Serial insertion of bilateral uncovered metal stents for malignant hilar obstruction using an 8 Fr biliary system: a case series of 17 consecutive patients
Introduction
There remains considerable debate over the need for unilateral versus bilateral stent placement in patients with malignant obstruction of the biliary hilum. Unilateral insertion of uncovered self-expanding metal stents (UCSEMS) for unresectable cholangiocarcinoma involving the hilum has been shown to be safe, feasible, and effective for the majority of patients (1,2). Placement of bilateral UCSEMS is technically challenging, and generally associated with lower rates of procedural success. Furthermore, metal stent insertion into both the right and left ductal systems appears to be comparable to unilateral insertion with regards to overall patency and complication-free survival, while increasing infectious complication rates (1-3).
Despite these potential advantages to unilateral UCSEMS, the goal of palliative stent placement in malignant biliary obstruction is to drain at least 50% of the liver volume (4,5). Drainage of more than half the liver volume often requires placement of more than one stent, resulting in bilateral or multi-segmental UCSEMS insertion. Previous studies examining the optimal technique in bilateral UCSEMS placement have reported significant technical limitations when stents are deployed in standard side-by-side fashion (6-8). Passing the second UCSEMS alongside the first stent is often impossible due to the radial expansive force from the previously deployed stent. However, with the development of thinner 6 Fr delivery catheters, two stents can be inserted through the working channel of the duodenoscope for bilateral, simultaneous deployment (6,9). Alternatively, the second stent may be deployed through the large interstices of the first stent using “stent-in-stent” technique (9), particularly when utilizing laser-cut (as opposed to woven) metal stents.
The WallflexTM uncovered biliary stent (Boston Scientific Inc., Marlborough, MA, USA) is a woven UCSEMS that utilizes an 8 Fr delivery catheter. The larger catheter size prevents simultaneous insertion of two stents down the endoscope channel. Also, the braided (woven) design of the stent creates smaller interstices, thus virtually preventing stent-in-stent deployment technique when placing bilateral stents. There are limited data, if any, on the technical success profile of this particular UCSEMS when used for bilateral palliation of malignant hilar obstruction. In the following report, we describe the feasibility and technical success, as well as procedural safety, in a series of 17 patients undergoing bilateral UCSEMS for malignant obstruction at the biliary hilum utilizing this 8 Fr delivery system.
Study design and endoscopic technique
This study was approved by the Stony Brook University Institutional Review Board (IRB). A retrospective review of our hospital’s endoscopy procedure software was performed between July 2008 and July 2014. Only ERCP cases in which bilateral UCSEMS were deployed for malignant hilar obstruction using the WallflexTM biliary stent were selected. The total number of attempted cases using the WallflexTM biliary stent was not included in this series, as this information is not routinely recorded in our electronic endoscopy records. Additional clinical data were gathered from the patients’ electronic medical record, including demographic characteristics, laboratory values, cancer treatment plans, and overall clinical course and hospitalizations. ERCP procedures were performed as clinically indicated after obtaining informed consent.
All procedures were performed by one of three endoscopists (JMB, SN, JCB) with anesthesia support. A standard therapeutic-channel video duodenoscope (models TJF-160 and TJF-180, Olympus America Inc., Center Valley, PA, USA) was used in each case. Biliary sphincterotomy was performed in all patients, or had been previously performed, before stent placement. Stricture dilation was utilized as needed for easier passage of the stent delivery catheter. Prophylactic intravenous antibiotics were given at the time of each procedure. Routine use of post-procedure antibiotics was not performed, unless the endoscopist noted inadequate drainage from a segment of liver that was filled with contrast.
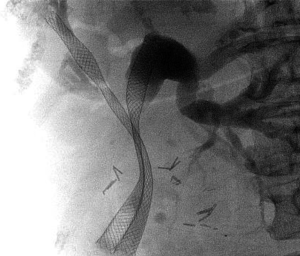
Following a diagnostic cholangiogram, two guidewires (0.035-inch HydraJagwire™ or Jagwire™, Boston Scientific Inc., Marlborough, MA, USA) were placed through the working channel of the duodenoscope and directed into the both the right and left ductal systems. These wires were positioned in side-by-side configuration. The hepatic duct with the more severe stenosis, or the more acute angle in take-off from the hilum, was chosen first for stent deployment. A preloaded 8 Fr delivery system was passed over this guidewire, alongside the second guidewire, and pushed up into the appropriate duct. The goal in choosing the proper stent size was to allow for at least 1 cm of patent stent above the stricture, while the distal end cross the ampullary orifice into the duodenum. The first stent was deployed completely alongside the existing, adjacent guidewire. After complete deployment of the first UCSEMS, the delivery catheter was removed leaving its guidewire in place. The second 8 Fr UCSEMS delivery system was then inserted over the second guidewire. This stent was gently pushed up into the other ductal system alongside the previously deployed stent. Some degree of endoscope position alteration was usually required to allow for complete advancement of the stent catheter across stricture while the first stent was already in place. Once the second UCSEMS was in the appropriate position, this stent was fully deployed with the aim of allowing the distal end to cross the ampullary orifice as seen in Figure 1. After fully deployed, the stent delivery catheter and both guidewires were removed.
Statistics
Descriptive statistics were reported as a mean value with standard deviation, or a median value with range, for all continuous variables. No additional statistical analyses were performed for this series.
Results and discussion
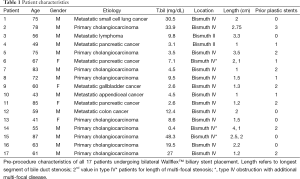
A total of 17 patients with malignant hilar obstruction were identified in which bilateral UCSEMS were inserted. The clinical characteristics of these 17 patients are listed in Table 1. Thirteen of 17 patients were male (76.5%). The median age was 63 years old (range, 41-87 years). Most patients had primary cholangiocarcinoma, Bismuth classification IV, as the cause of their biliary obstruction. Metastatic cancers due to non-biliary malignancies were the causes of obstruction in the remaining 8/17 patients. All patients who were stented were at the initial phases of their chemotherapy regimens. All patients presented with obstructive jaundice and pruritus.
Full table
The mean total bilirubin level prior to undergoing any biliary intervention was 13.4±13.7 mg/dL (Table 1). The mean length of the longest segment of biliary stricture was 1.5±0.8 cm. Three patients (17.6%) had multi-focal disease, with additional strictures either above or below the hilum. Eleven of 17 patients (64.7%) had an initial ERCP with plastic stent placement prior to UCSEMS insertion; median number of stents placed was 2 (range, 1-3).
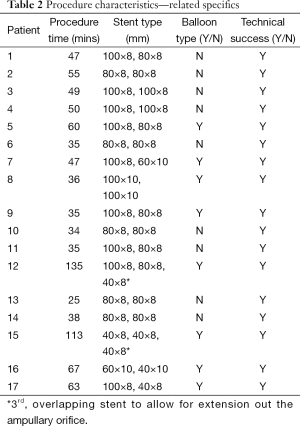
Procedure characteristics are shown in Table 2. The average procedure time was 54.4 minutes (range, 35-135 minutes). In 8/17 patients (47.1%), balloon dilation of the stricture was performed prior to UCSEMS placement. In all but two patients, both biliary stents were successfully deployed with their distal ends crossing the ampullary orifice within the duodenum. In both of these patients, a 3rd uncovered 40 mm × 8 mm UCSEMS was inserted over the 2nd guidewire to allow for adequate transpapillary drainage. All procedures were technically successful. There were no episodes of stent deployment malfunction in these 17 cases. One patient required initial placement of a percutaneous transhepatic biliary drain prior to successful ERCP because of failed retrograde biliary cannulation. The drain was removed at the time of the 2nd ERCP in which bilateral UCSEMS were inserted.
Full table
Nine of 17 patients (52.9%) required inpatient hospitalization following their elective outpatient procedure. The reasons for admission were post-procedure abdominal pain and the need for intravenous antibiotics to prevent cholangitis (due inadequate drainage of contrast from one segment of liver). There was only one case of post-procedure cholangitis treated successfully with antibiotics. This patient also required subsequent percutaneous transhepatic biliary drain placement due to persistent hyperbilirubinemia. There were no episodes of post-procedure stent obstruction, pancreatitis, perforation, bleeding, or other infections aside from cholangitis in this series. There were no deaths directly related to the ERCP procedure. Post-procedure bilirubin levels 2 weeks after stent insertion were not universally available. However, at least 10/17 patients (58.8%) were seeing an oncologist for palliative chemotherapy after stent placement, suggesting adequate biliary drainage. The remaining seven patients elected for hospice care only, or were lost to follow-up.
Biliary drainage for unresectable malignant hilar obstruction is challenging and frequently performed by the insertion of UCSEMS, in an attempt to reduce hyperbilirubinemia so that patients may undergo palliative chemotherapy. Though controversy exists over unilateral versus bilateral stenting, the placement of bilateral stents is often necessary to drain more than half the liver volume and provide the best opportunity for improvement in hepatic function. With the advent of new and improved biliary endoprostheses, the technical feasibility of bilateral stenting has become somewhat less challenging for the biliary endoscopist.
Following the recent introduction of the WallflexTM biliary UCSEMS into clinical practice, there have been limited data describing the use of this particular stent for bilateral palliation of malignant hilar obstruction. In 2012, Luigiano and colleagues (10) were the first to specifically compare the WallflexTM UCSEMS with its predecessor UCSEMS, the biliary Wallstent™. Although late adverse events appeared to be lower in the WallflexTM group (10.3% vs. 27.8%), only a small proportion of patients in this study had hilar obstruction (12% WallflexTM and 4% Wallstent™), and there was no report of bilateral WallflexTM insertion (10). Almadi and colleagues recently performed a thorough meta-analysis examining the use of covered and uncovered SEMS for biliary obstruction, many of which utilized the WallflexTM stent (11). However, this study was designed to focus on distal biliary obstruction, and bilateral placement across the hilum was not addressed.
Earlier studies that examined the feasibility of bilateral biliary stenting specifically in hilar malignancies focused mainly on biliary stents with smaller delivery systems, such as the 6 Fr Zilver 635™ UCSEMS (Cook Medical Inc., Winston Salem, NC, USA), or the 7 Fr Fusion Zilver™ UCSEMS (Cook Medical Inc., Winston Salem, NC, USA). In 2009, Kim et al. (12) reported a technical success rate of 85.3% (29/34) using the stent-in-stent technique with the 7 Fr Zilver™ delivery system in conjunction with the Niti-S™ Biliary Y stent (Taewoong Medical Co., South Korea). It was suggested that the Zilver’s slimmer 7 Fr delivery system is what allowed for this UCSEMS to be inserted through the central portion of the Y stent into the contralateral hepatic duct. Chennat et al. (6) conducted a small case series of 16 patients examining side-by-side simultaneous deployment of the 6 Fr delivery system Zilver™ biliary stent with a success rate of 62.5% (10/16). However, the bilateral hilar stents did not bridge the ampullary region, and so all of these patients required additional stent placement to achieve transpapillary drainage.
In this brief report, we demonstrate our initial results regarding the feasibility, technical success, and early procedural safety of bilateral uncovered WallflexTM insertion for malignant hilar strictures. Bilateral stenting was successfully achieved in less than 1 hour (average time 54 minutes) in all 17 patients; keeping in mind that the total number of attempted cases was not reported. Though the rate of inpatient hospitalization is somewhat higher compared to the early complication rates reported in prior studies [53% vs. 27-41% (3,9)], the majority of post-procedure admissions were due to transient abdominal pain rather than procedure-related complications (i.e., pancreatitis, hemobilia, liver abscess, stent obstruction, etc.). Only one patient developed procedural-induced cholangitis; but this patient was treated successfully with IV antibiotics and no further clinical consequences. Although the WallflexTM UCSEMS utilizes a larger 8 Fr delivery catheter with smaller interstices than other available stents, the inability to perform traditional “side-by-side” or “stent-in-stent” techniques (9) did not prevent successful insertion or cause deployment malfunction. Serial or subsequent stent placement was not limited by the radial force of the first stent deployed. The reason for this is likely related to the flexible nature of the completely deployed first stent. Furthermore, although not unique to this stent, its looped and flared ends may limit—to some degree—the amount of stent migration that occurs when pushing the second stent catheter into position before deployment. Lastly, many of our patients had previous plastic stents (64.7%) or balloon dilation (47.1%) prior to UCSEMS insertion. Some or all of these factors may account for the high degree of technical success observed in this series.
There are clearly limitations and inherent biases with this retrospective case series that warrant mention. As noted above, many of the patients had prior plastic stents inserted, and this may have facilitated the ease of insertion of either the first or the second 8 Fr delivery catheter. Furthermore, balloon dilation of the stricture was not uniformly performed in each patient, and pre-dilation of a stricture also affects the ease of subsequent stent insertion. In addition, there was lack of standardization in the diameter sizes of each stent inserted, as well as heterogeneity in the different types of malignant hilar obstruction intervened upon. Each of these factors may affect the technical success rate, and therefore the safety profile, of bilateral UCSEMS placement. Finally, long-term clinical outcomes were not addressed as many of the patients were discharged after their procedures with follow-up at outside institutions.
In conclusion, there are limited data describing the use of the 8 Fr delivery WallflexTM stent for the treatment of hilar biliary obstruction, and no prior reports that specifically examine the bilateral insertion of this endoprosthesis. This case series illustrates that decompression of malignant hilar biliary obstruction can be reasonably achieved by means of bilateral insertion of UCSEMS using these biliary stents. Serial placement of this device into the right and left systems can be performed with good technical feasibility and safety profiles. Despite its larger delivery size, the unique design of this stent appears to enable the biliary endoscopist to overcome the difficulties traditionally encountered in deploying bilateral stents within anatomically complex locations.
Acknowledgements
None.
Footnote
Conflicts of Interest: Juan Carlos Bucobo and Jonathan M. Buscaglia are consultants and speakers for Boston Scientific, Inc.
Ethical Statement: This study was approved by the Stony Brook University Institutional Review Board. ERCP procedures were performed as clinically indicated after obtaining informed consent.
References
- De Palma GD, Galloro G, Siciliano S, et al. Unilateral versus bilateral endoscopic hepatic duct drainage in patients with malignant hilar biliary obstruction: results of a prospective, randomized, and controlled study. Gastrointest Endosc 2001;53:547-53. [PubMed]
- De Palma GD, Pezzullo A, Rega M, et al. Unilateral placement of metallic stents for malignant hilar obstruction: a prospective study. Gastrointest Endosc 2003;58:50-3. [PubMed]
- Iwano H, Ryozawa S, Ishigaki N, et al. Unilateral versus bilateral drainage using self-expandable metallic stent for unresectable hilar biliary obstruction. Dig Endosc 2011;23:43-8. [PubMed]
- Rerknimitr R, Angsuwatcharakon P, Ratanachu-ek T, et al. Asia-Pacific consensus recommendations for endoscopic and interventional management of hilar cholangiocarcinoma. J Gastroenterol Hepatol 2013;28:593-607. [PubMed]
- Lee TH. Technical tips and issues of biliary stenting, focusing on malignant hilar obstruction. Clin Endosc 2013;46:260-6. [PubMed]
- Chennat J, Waxman I. Initial performance profile of a new 6F self-expanding metal stent for palliation of malignant hilar biliary obstruction. Gastrointest Endosc 2010;72:632-6. [PubMed]
- Dumas R, Demuth N, Buckley M, et al. Endoscopic bilateral metal stent placement for malignant hilar stenoses: identification of optimal technique. Gastrointest Endosc 2000;51:334-8. [PubMed]
- Cheng JL, Bruno MJ, Bergman JJ, et al. Endoscopic palliation of patients with biliary obstruction caused by nonresectable hilar cholangiocarcinoma: efficacy of self-expandable metallic Wallstents. Gastrointest Endosc 2002;56:33-9. [PubMed]
- Law R, Baron TH. Bilateral metal stents for hilar biliary obstruction using a 6Fr delivery system: outcomes following bilateral and side-by-side stent deployment. Dig Dis Sci 2013;58:2667-72. [PubMed]
- Luigiano C, Ferrara F, Cennamo V, et al. A comparison of uncovered metal stents for the palliation of patients with malignant biliary obstruction: nitinol vs. stainless steel. Dig Liver Dis 2012;44:128-33. [PubMed]
- Almadi MA, Barkun AN, Martel M. No benefit of covered vs uncovered self-expandable metal stents in patients with malignant distal biliary obstruction: a meta-analysis. Clin Gastroenterol Hepatol 2013;11:27-37. [PubMed]
- Kim JY, Kang DH, Kim HW, et al. Usefulness of slimmer and open-cell-design stents for endoscopic bilateral stenting and endoscopic revision in patients with hilar cholangiocarcinoma (with video). Gastrointest Endosc 2009;70:1109-15. [PubMed]