Feasible usage of ABO incompatible grafts in living donor liver transplantation
Introduction
Although living donor liver transplantation (LDLT) has now become a treatment of option for patients with end-stage liver disease, especially in eastern countries, its application is limited by the need for an appropriate living donor (1,2). Under these circumstances, ABO incompatible (ABOi) LDLT has been practiced in Japan (3-9).
Despite the dismal outcomes in the initial series, the application of local infusion (LI) treatment, which delivers protease inhibitors, prostaglandin and steroids via the portal vein (PV) or hepatic artery (HA), increased the survivals of ABOi-LDLT (3,4). Nevertheless, such LI are associated with very high incidence of catheter-associated problems including hepatic hilar vascular thrombosis, or intra-abdominal bleeding (4).
Rituximab is an anti-CD20 monoclonal antibody that specifically targets the CD20 surface antigen expressed on B-lymphocytes (5,6). It has been shown to induce complement-mediated cell lysis on CD20 positive cells (5,6). In 2005, we have introduced the use of rituximab in ABOi-LDLT, and abandoned the use of LI since 2006, under the use of high-dose intravenous immunoglobulin (HD-IVIG), with acceptable short-term graft survival rate over 90% (7). Since then, we have started to reserve IVIG for actual treatment for antibody mediated rejection (AMR) and reserved the use of it since 2009 (8).
In the current article, we reviewed our strategies to have successful ABOi-LDLT and the actual outcomes and pitfalls.
Materials and methods
Patients
Between May 1997 and March 2013, 408 LDLTs in adults were performed at Kyushu University Hospital, Japan. Among them, 19 patients received ABOi-LDLTs. All the LDLTs were performed after obtaining full informed consent from all patients and approval by the Liver Transplantation Committee of Kyushu University. The basic surgical procedures and techniques were described previously (10,11). All 19 patients received duct-to-duct biliary reconstruction. All patients received pneumococcal vaccination a month before LDLT. The mean follow-up period was 5.1±2.1 years.
Basic immunosuppression regimen
The basic immunosuppression induction regimen in ABOi LDLT involved the administration of tacrolimus with mycophenolate mofetil and steroids. Currently, mycophenolate mofetil is started 7 days before LDLT at a dose of 2 g/day, and increased to 3 g/day after LDLT, then decreased to 2 g/day once the blood calcineurin inhibitor level reaches an appropriate level. Tacrolimus is started within 3 days after LDLT once the kidney function has recovered. The target Tacrolimus level ranges between 12 to 15 ng/mL for the first post-LDLT month and is titrated down to 8 to 10 ng/mL for the next few months. When patients experience tacrolimus associated complications, especially encephalopathy, Tacrolimus is converted to cyclosporine A. The target cyclosporine A level ranges from 200 to 250 ng/mL for the first post-LDLT month and was titrated down to 100 to 150 ng/mL for the next few months. A gram of methylprednisolone is given after reperfusion, and tapered from 200 to 40 mg over 10 days, then switched to 20 mg of oral prednisolone and tapered off in 6 months after the LDLT.
Plasma exchange (PE)
PE was performed using 40 U of fresh frozen plasma to lower the isoagglutinin titer ≤128 before LDLT. PE was also indicated after LDLT if a patient showed clinical presentation of AMR with elevated liver enzymes with progressive increase in isoagglutinin titers (12). Post-LDLT PE was performed when daily progressive elevation of isoagglutinin titer, indicating highly possible AMR, was observed.
Local infusion (LI)
LI via the PV was exclusively used between 2001 and 2005 (13). A 16 G double lumen catheter was introduced from the umbilical vein or the mesenteric vein and protease inhibitor (Nafamostat Mesilate, 200 mg/day), prostaglandin E1 (500 micro g/day) and methylprednisolone (50 mg/day) were given for 14 days after LDLT. PEs were performed to lower the isoagglutinin titer ≤64. Since the case #4 in 2006, LI was abandoned because of catheter-associated complications in 2 of 3 cases (7).
Rituximab
Rituximab (375 mg/m2) was given has been administered since the case #3 in 2005. Although both rituximab and LI were used for the case #3, rituximab-based desensitization protocol was used since then (7).
Rituximab was administered under pretreatment using 100 mg of hydrocortisone. The timing of the administration is scheduled 3 weeks before LDLT for scheduled cases with chronic liver diseases. The absence of blood CD20 was confirmed using flow cytometry at least 2 weeks before LDLT. For the cases with acute liver failure (ALF), rituximab was intravenously given as soon as the indication of emergent LDLT was confirmed after donor and recipient work-ups (12) (Figure 1).
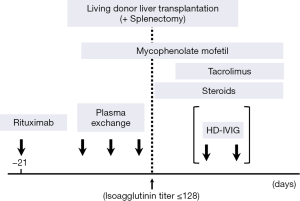
High-dose intravenous immunoglobulin (HD-IVIG)
Since 2006 when LI was abandoned, HD-IVIG (0.6−1.0 g/kg) given on day 0 and 4 after LDLT was used in combination with rituximab as a prophylaxis manner for AMR (7). When it was used, continuous hemodiafiltration (CHDF) was simultaneously applied to remove the high fluid volume of HD-IVIG to prevent pulmonary edema.
Since 2009, only rituximab was used to desensitization, and HD-IVIG has been reserved for treating actual AMR (8) (Figure 1).
Antibody-mediated rejection (AMR)
We define AMR as histological acute humoral rejection was suspected when peri-portal hemorrhagic edema associated with the progressive daily elevation of isoagglutinin titers and liver enzymes.
Statistical analyses
All analyses were conducted in a retrospective manner. Values are expressed as the mean ± standard deviation (SD). Variables were analyzed using χ2 tests for categorical values or Mann-Whitney tests for continuous variables. Cumulative survival analyses were determined using the Kaplan-Meier method with the log-rank test. Values of P<0.05 were considered statistically significant.
Results
Donor and recipient data
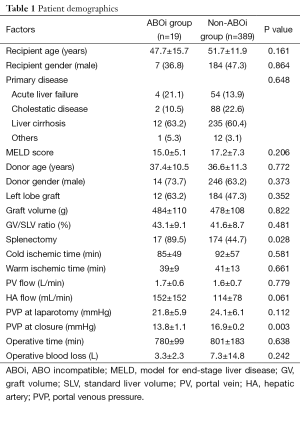
The patients who received ABOi-LDLT had mean recipient age of 47.7±15.7 years and had seven males (36.8%). Primary liver disease for LDLT included ALF (n=4), hepatitis B with hepatocellular carcinoma (n=3), hepatitis C with hepatocellular carcinoma (n=8), liver cirrhosis due to alcohol overconsumption (n=1), primary biliary cirrhosis (n=1), primary sclerosing cholangitis (n=1), and giant hemangioma (n=1). The mean model for end-stage liver disease (MELD) score was 15.0±5.1. There was no statistically significant difference between ABOi and non-ABOi-group in the recipient background factors (Table 1). Blood type combinations between the donors and recipients included A to O (n=4), A to B (n=6), B to A (n=3), B to O (n=1), AB to B (n=4) and AB to A (n=1).
Full table
The mean age of the donors for ABOi-LDLT was 37.4±10.5 years and had 14 males (73.7%). The graft types included extended left lobe graft with middle haptic vein and caudate lobe (n=12), right lobe graft (n=6), and posterior segment graft (n=2). The mean graft volume (GV) and GV/standard liver volume ratio (GV/SLV) was 484±110 g and 43.1%±9.1%, respectively. There was no statistically significant difference between ABOi and non-ABOi-group in the donor background factors (Table 1).
In ABOi-LDLT group, the frequency of splenectomy was significantly increased (89.4% vs. 44.7%, P=0.028), and the portal venous pressure (PVP) at the end of surgery was significantly lower (13.8±1.1 vs. 16.9±0.2 mmHg, P=0.003). Other surgical factors including warm/cold ischemic time, portal or hepatic arterial flow after reperfusion, PVP at laparotomy, operative time and blood loss, were not different between the groups (Table 1).
Isoagglutinin titers and CD20 positive cells.
PE effectively lowered the isoagglutinin titers before LDLT in all the cases ≤128, except one case with isoagglutinin titer of 512 at LDLT. The patient received two session of HD-IVIG after LDLT for prophylaxis treatment for AMR. There were two patients who experienced rebound elevation of the isoagglutinin titers over 1,024 times with clinical AMR. They were patients with ALF and received rituximab 3 days before LDLT. They received session of PEs with or without IVIG and successfully treated (Figure 2).
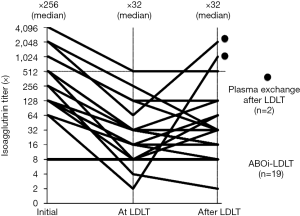
The patients since the case #3 in 2005 received rituximab before LDLT and CD20 positive lymphocytes were totally suppressed at the time of LDLT after administration of rituximab.
Surgical outcomes and graft survivals
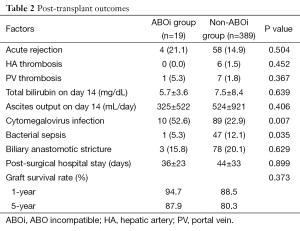
In ABOi-LDLT group, the patients had more increased incidence of cytomegalovirus antigenemia (52.6% vs. 22.9%, P=0.007) but decreased incidence of bacterial sepsis (5.3% vs. 12.1%, P=0.035) (Table 2). There were no significant differences in post-surgical complications including acute rejection, hepatic arterial or portal venous thrombosis, and biliary anastomotic stricture. The total bilirubin level and ascites output from abdominal drain in ABOi-LDLT group was 5.7±3.6 and 325±522 mL respectively, and were not difference from those in non-ABOi-LDLT group (7.5±8.4 and 524±921 mL, respectively). The mean post-LDLT hospital stay was 36±23 days in ABOi-LDLT group and 44±33 days in non-ABOi-LDLT group (P=0.899).
Full table
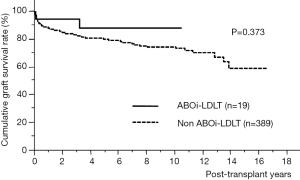
The 1- and 5-year cumulative graft survival rate was 94.7% and 87.9% in ABOi-LDLT group and 88.5% and 80.3% in non-ABOi-group, respectively without any significant differences (P=0.373) (Figure 3). In ABOi-group, graft loss occurred in two patients; one from diffuse portal venous thrombosis and graft necrosis due to LI, and the other from chronic rejection presented as veno-occlusive disease at 3 years after LDLT.
Discussion
Despite the unsatisfactory outcomes in ABOi-LDLT, trials for improving its survival rate have been undertaken in Japan, where there is a minimal chance to acquire liver grafts from deceased donors (1-9,14). The first significant advance in this field was the application of LI treatment, including the intra-portal or intra-arterial infusion of prostaglandin E1, mesylate gabexate, and methylprednisolone (3). The rationale for prostaglandin and mesylate gabexate is for treating disseminated coagulation induced by blood type antigen-antibody reaction (15). Although this improved the results after ABOi-LDLT, the problems encountered included catheter associated thrombosis and the need of re-laparotomy for the removal of the catheters (4). Our experience with this approach included the complications of catheter associated PV thrombosis and hepatic arterial dissection. Thereafter, LI treatment was abandoned in our center (7). However, it was also true that LI treatment pushed up the graft survival rate after ABOi-LTLT over 50% (4).
Rituximab, an anti-CD20 antibody, is a monoclonal antibody that specifically targets the CD20 surface antigen expressed on-B lymphocytes, thus resulting in cell lyses (16). Although rituximab might totally control AMR over blood type barriers, it does not do so because it cannot eradicate plasma cells (17). Egawa et al. (17) has reported that administration of rituximab earlier than 7 days before LDLT significantly depleted CD20 positive B- and memory B-lymphocytes and lowered the peak post-LDLT isoagglutinin titers. Usui et al. (5) reported on its use as long as 3 weeks before the LDLT with successful outcomes. They revealed that not only B-cells but also plasma cells were depleted when rituximab was administered 3 weeks before LDLT (5).
However for cases with ALF, administration of rituximab 3 weeks before LDLT was impossible. Therefore, we applied HD-IVIG as the new immunomodulation protocol in ABOi-LDLT (7). Actually in the field of kidney transplantation, the effective use of IVIG for the control of acute humoral rejections in highly sensitized candidates was utilized (18-20). The proposed mechanisms of action of IVIG on the humoral reaction include B-cell or plasma cell apoptosis through the Fc-receptor dependent pathway, and the inhibition of alloreactive T-cell mediated or complement-mediated allograft injury, although these possibilities have not been confirmed (18-20). HD-IVIG might have worked on for these plasma cells, preventing AMR. However, the most significant problems in the use of HD-IVIG is its high cost, and thus we reserve the use of HD-IVIG as rescue for actual AMR and prophylaxis administration is held. Regarding the dosage of rituximab, no definite consensus has been established yet. However, Egawa et al. (14) recently reported that regular single dose of rituximab (500 mg/body or 375 mg/m2) had lower incidence of AMR than single smaller dose (300 mg/body), and multiple dosage of rituximab significantly increased the incidence of fungal and viral infectious episodes.
For patients with ALF, we are trying to put rituximab 2 weeks before LDLT and keeping the patients away from the progression of hepatic encephalopathy, coma and brain death, using high-flow CHDF (HF-CHDF). HF-CHDF is a mode of CHDF and uses much more volumes of buffer as much as 200 L and it efficiently removes more low and middle molecular weight toxic substances (21-23). Yokoi et al. (21) evaluated the clinical efficacy of HF-CHDF for treating patients with those of conventional treatments without HF-CHDF, and found that recovery from coma was significantly improved in the HF-CHDF group. Nevertheless, we think that IVIG is the last hope of treatment if encephalopathy were not controlled even by HF-CHDF and emergent LDLT could not be avoided (7).
Regarding the role of splenectomy in ABOi-LDLT, our standpoint is splenectomy is unnecessary if LDLT is performed 2 to 3 weeks after Rituximab administration and not only CD20 cells but also plasma cells were depleted as Kyoto group reported (24). However, for the emergent cases in which Rituximab was given just several days before LDLT and HF-CHDF cannot effectively treat progressive encephalopathy, splenectomy is necessary although splenectomy associated surgical complications are warranted (25). We have previously showed that a cause who received Rituximab several days before ABOi-LDLT had CD138 positive plasma cells in spleen (7).
In conclusion, ABOi-LDLT could be safely performed, especially under Rituximab-based protocol.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: LDLTs were performed after obtaining full informed consent from all patients and approval by the Liver Transplantation Committee of Kyushu University.
References
- Taketomi A, Kayashima H, Soejima Y, et al. Donor risk in adult-to-adult living donor liver transplantation: impact of left lobe graft. Transplantation 2009;87:445-50. [PubMed]
- Ikegami T, Shirabe K, Soejima Y, et al. Strategies for successful left-lobe living donor liver transplantation in 250 consecutive adult cases in a single center. J Am Coll Surg 2013;216:353-62. [PubMed]
- Tanabe M, Shimazu M, Wakabayashi G, et al. Intraportal infusion therapy as a novel approach to adult ABO-incompatible liver transplantation. Transplantation 2002;73:1959-61. [PubMed]
- Egawa H, Teramukai S, Haga H, et al. Present status of ABO-incompatible living donor liver transplantation in Japan. Hepatology 2008;47:143-52. [PubMed]
- Usui M, Isaji S, Mizuno S, et al. Experiences and problems pre-operative anti-CD20 monoclonal antibody infusion therapy with splenectomy and plasma exchange for ABO-incompatible living-donor liver transplantation. Clin Transplant 2007;21:24-31. [PubMed]
- Egawa H, Ohdan H, Haga H, et al. Current status of liver transplantation across ABO blood-type barrier. J Hepatobiliary Pancreat Surg 2008;15:131-8. [PubMed]
- Ikegami T, Taketomi A, Soejima Y, et al. Rituximab, IVIG, and plasma exchange without graft local infusion treatment: a new protocol in ABO incompatible living donor liver transplantation. Transplantation 2009;88:303-7. [PubMed]
- Soejima Y, Muto J, Matono R, et al. Strategic breakthrough in adult ABO-incompatible living donor liver transplantation: preliminary results of consecutive seven cases. Clin Transplant 2013;27:227-31. [PubMed]
- Kozaki K, Egawa H, Ueda M, et al. The role of apheresis therapy for ABO incompatible living donor liver transplantation: the Kyoto University experience. Ther Apher Dial 2006;10:441-8. [PubMed]
- Ikegami T, Shirabe K, Yamashita Y, et al. Small upper midline incision for living donor hemi-liver graft procurement in adults. J Am Coll Surg 2014;219:e39-43. [PubMed]
- Ikegami T, Soejima Y, Taketomi A, et al. Explanted portal vein grafts for middle hepatic vein tributaries in living-donor liver transplantation. Transplantation 2007;84:836-41. [PubMed]
- Ikegami T, Taketomi A, Soejima Y, et al. Successful ABO incompatible living donor liver transplantation in a patient with high isoagglutinin titer using high-dose intravenous immunoglobulin. Transplant Proc 2007;39:3491-4. [PubMed]
- Suehiro T, Shimada M, Kishikawa K, et al. Effect of intraportal infusion to improve small for size graft injury in living donor adult liver transplantation. Transpl Int 2005;18:923-8. [PubMed]
- Egawa H, Teramukai S, Haga H, et al. Impact of rituximab desensitization on blood-type-incompatible adult living donor liver transplantation: a Japanese multicenter study. Am J Transplant 2014;14:102-14. [PubMed]
- Demetris AJ, Jaffe R, Tzakis A, et al. Antibody-mediated rejection of human orthotopic liver allografts. A study of liver transplantation across ABO blood group barriers. Am J Pathol 1988;132:489-502. [PubMed]
- Pescovitz MD. Rituximab, an anti-cd20 monoclonal antibody: history and mechanism of action. Am J Transplant 2006;6:859-66. [PubMed]
- Egawa H, Ohmori K, Haga H, et al. B-cell surface marker analysis for improvement of rituximab prophylaxis in ABO-incompatible adult living donor liver transplantation. Liver Transpl 2007;13:579-88. [PubMed]
- Sonnenday CJ, Warren DS, Cooper M, et al. Plasmapheresis, CMV hyperimmune globulin, and anti-CD20 allow ABO-incompatible renal transplantation without splenectomy. Am J Transplant 2004;4:1315-22. [PubMed]
- Jordan SC, Vo AA, Peng A, et al. Intravenous gammaglobulin (IVIG): a novel approach to improve transplant rates and outcomes in highly HLA-sensitized patients. Am J Transplant 2006;6:459-66. [PubMed]
- Glotz D, Antoine C, Julia P, et al. Desensitization and subsequent kidney transplantation of patients using intravenous immunoglobulins (IVIg). Am J Transplant 2002;2:758-60. [PubMed]
- Yokoi T, Oda S, Shiga H, et al. Efficacy of high-flow dialysate continuous hemodiafiltration in the treatment of fulminant hepatic failure. Transfus Apher Sci 2009;40:61-70. [PubMed]
- Kubota T, Sekido H, Takeda K, et al. Acute hepatic failure with deep hepatic coma treated successfully by high-flow continuous hemodiafiltration and living-donor liver transplantation: a case report. Transplant Proc 2003;35:394-6. [PubMed]
- Inoue K, Watanabe T, Hirasawa H, et al. Liver support systems as perioperative care in liver transplantation-historical perspective and recent progress in Japan. Minerva Gastroenterol Dietol 2010;56:345-53. [PubMed]
- Raut V, Mori A, Kaido T, et al. Splenectomy does not offer immunological benefits in ABO-incompatible liver transplantation with a preoperative rituximab. Transplantation 2012;93:99-105. [PubMed]
- Wang H, Ikegami T, Harada N, et al. Optimal changes in portal hemodynamics induced by splenectomy during living donor liver transplantation. Surg Today 2015;45:979-85. [PubMed]


