Retinoid roles in blocking hepatocellular carcinoma
Retinoid roles in liver disease leading to hepatocellular carcinoma (HCC)
Chronic liver damage leads progressively to liver fibrosis, liver cirrhosis, and HCC, regardless of etiology, whether due to chronic hepatitis B and/or C virus (HBV and/or HCV) infection, excessive and chronic alcohol consumption, iron overload, dietary exposure to aflatoxin B1, or non-alcoholic fatty liver disease/non-alcoholic steatohepatitis (NAFLD/NASH) (1-3). Consequently, the best strategy for blocking HCC development is to treat early and to prevent further exacerbation of chronic hepatitis by appropriate treatments for the underlying cause of hepatic disease; for instance, using anti-viral therapy, including interferon (IFN), reverse-transcriptase inhibitors, and direct-acting antiviral agents, for chronic HBV/HCV infection or possibly using anti-diabetic medications for NAFLD/NASH.
The evidence pointing to a role for retinoids in preventing liver disease development comes from cell culture studies, animal model studies and human studies. This information will be reviewed briefly below. Some of the most compelling data regarding retinoids and liver disease has been obtained from the study of induced mutant mice. Table 1 provides a listing of studies in mutant mice that establish linkages between retinoid metabolism and actions and liver disease.

Full table
It has been well established that transcriptional activity of the retinoic acid isomers, all-trans-retinoic acid (ATRA) and 9-cis-retinoic acid (9cRA) regulates expression of a number of genes associated with fat metabolism (7,8). The transcriptional regulatory actions of ATRA and 9cRA are mediated by nuclear hormone receptors, including three distinct retinoic acid receptors (RARα, −β, and −γ) and three distinct retinoid X receptors (RXRα, −β, and −γ). Transgenic mice, which express an RARα-dominant negative form in hepatocytes, exhibit rapid spontaneous development of steatohepatitis (4). Expression of the RARα-dominant-negative form was proposed to suppress the activities of endogenous RAR/RXR heterodimers (9), resulting in the down-regulation of hepatic mitochondrial β-oxidation and an up-regulation of peroxisomal β-oxidation. The authors also observed spontaneous development of liver tumors, including HCC, in the mice after reaching 12 months of age. Interestingly, by feeding high levels of retinoic acid to the mice, histologically identifiable disorders in the liver, such as steatosis, were ameliorated and the incidence of liver tumors decreased.
A variety of other published studies establish relationships between retinoids and liver disease. Studies of mutant mice lacking β-carotene-15'15'-monooxygenase (BCO1) (5), the sole mammalian enzyme responsible for cleaving provitamin A carotenoids to retinoids, have demonstrated that these mutant mice develop a fatty liver and are more susceptible than control mice to high fat diet-induced impairments in fatty acid metabolism. This suggests that BCO1 has a role in modulating hepatic fat metabolism. Oxidative stress induced by chronic inflammation plays a key role in the development of chronic liver disease and HCC (10). The loss of hepatic retinoid signaling has been associated with more rapid progression of liver disease development arising from reactive oxygen species (11). Ashla et al. (12) performed hepatic gene expression profiles of genes involved in retinoid metabolism in NAFLD patients to investigate mechanisms underlying NAFLD/NASH development. This analysis indicated that a hyperdynamic state of retinoid metabolism occurs in the livers of NAFLD patients. When taken together, these published findings indicate that retinoids have important roles in mediating normal lipid metabolism in the liver and that altered retinoid signaling is associated with the progression of NAFLD/NASH.
With reference to NAFLD/NASH as hepatic manifestations of metabolic syndrome, diabetes mellitus and dyslipidemia must also be regarded as a risk factors leading to HCC development (13). RXRs form heterodimers with other nuclear hormone receptors such as peroxisomal proliferator-activated receptors (PPARs) and liver X receptors (LXRs) (14). PPARγ/RXR is known to be a target of thiazolidinediones (TZDs) (15), agents used clinically for treating diabetes and improving insulin resistance, and LXR/RXR is involved in regulating glucose and lipid metabolism (16). These heterodimers involving RXR are able to be activated by RXR agonists alone and this is referred to as a permissive mechanism (17). Although RXR agonists were considered to be effective and useful for treating metabolic syndrome, the existing RXR agonists also have adverse effects in patients resulting in toxicities (18,19). Therefore, RXR partial agonists developed by systematic chemical conversions of full agonists have been synthesized and validated for clinical usage. There are expected to show sufficient efficacy against diabetes and hyperlipidemia without severe side effects (20). A study focusing on insulin resistance, which is fundamental to diabetes mellitus development, established that oral administration of ATRA significantly improved insulin sensitivity in a diet-induced mouse model of NAFLD in the setting of simultaneous genetically-induced insulin resistance (21). Since the effect of ATRA for ameliorating insulin resistance was not observed in mutant mice lacking leptin and ATRA markedly elevated leptin receptor expression in the livers of NAFLD mice, these data establish that retinoids may improve insulin sensitivity in a leptin-dependent manner.
Recent studies have also reported the important role of retinol-binding protein 4 (RBP4) in NAFLD and insulin resistance. RBP4, initially known as a transport protein for the delivery of retinol through the circulation, has been identified as a novel adipokine that is proposed to link obesity, insulin resistance and impaired glucose tolerance (3,22,23). Insulin resistance is observed in transgenic overexpressing human RBP4, as well as upon injection of recombinant RBP4 into the circulations of normal mice (6), while RBP4 knockout mice show improved insulin sensitivity compared to normal mice (24). In obese and diabetic individuals, serum levels of RBP4 are reported to be elevated over those of healthy individuals (22). Even in non-diabetic individuals, RBP4 is increased in NAFLD patients (23). Therefore, it is thought that RBP4 may play a significant role in NAFLD. Xia et al. (25) have reported a novel mechanism by which RBP4 exerts its effects on lipid metabolism in the liver; treatment with human retinol bound RBP4 up-regulated the levels of sterol regulatory element-binding protein 1 (SREBP-1), followed by enhancing the expression of lipogenic genes, including fatty acid synthase (FAS) and acetyl coenzyme A carboxylase-1 (ACC-1), leading to increased intracellular triglyceride synthesis in HepG2 cells.
Chronic HCV infection is a major cause of liver cirrhosis and HCC. Although newly-developed direct-acting antiviral agents are becoming available, IFN still remains the most important agent used clinically against HCV infection. Retinoids have been reported to enhance the anti-cancer effect of IFN in several tumor cell lines (26,27). In an in vitro study employing ATRA and 9cRA treatments of cells in culture, expression levels of the IFN receptor in the cell lines were found to be elevated upon treatment with either ATRA or 9cRA (28). Based on these cell culture results, a cohort study was undertaken to study the combined effects of ATRA and IFN treatment in HCV patients. This study demonstrated a strong additive combinational effect of ATRA and IFN for therapy against HCV infection (29). Pointing to the same conclusion, Bitetto et al. (30) recently reported that a high percentage of patients suffering from chronic HCV infection showed lower serum vitamin A levels that were classified as being deficient, and that the deficiency was associated with less responsiveness to IFN-based anti-viral therapy.
Acyclic retinoid (ACR) for blocking HCC: experimental studies
ACR is a synthetic retinoid that is an agonist for both RARs and RXRs (31). It has been shown in experimental studies that this retinoid has several beneficial effects on blocking HCC development. Muto and Moriwaki reported that ACR suppresses chemically-induced liver tumors and spontaneous HCC development in rodents (32). Through in vitro analysis, these investigators found that ACR induces apoptosis and inhibits cellular proliferation in human hepatoma cell lines. This effect on hepatoma cells was shown to be mediated through induction of cellular differentiation and apoptosis; ACR arrests cell cycle in the G0-G1 phase, increases levels of p21 protein, which then negatively regulates cell cycle progression, and decreases levels of the cyclin D1 (33). These investigators also established that ACR suppresses Ras/MAP kinase signal transduction and restores the functionality of RXRα by reducing levels of phosphorylated RXRα, which accumulates in the nucleus (34) and promotes HCC growth due to interfering with normal RXRα functions (35).
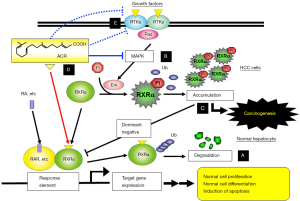
Other studies, involving cell lines and animals, have demonstrated that ACR suppresses HCC development and modulates cancer cell growth by inhibiting the activation of receptor tyrosine kinases (RTKs), which play an important role in stimulating of the Ras/MAP kinase signaling pathway (22,36-38). Thus, ACR causes direct inhibition of the Ras/Erk signaling system (35) as well as dephosphorylation of Erk and RXRα proteins due to its inactivating effects on RTKs. Moreover, it has been reported that ACR controls HCC development by suppressing transforming growth factor α (TGFα) expression (39), by modulating both fibroblast growth factor signaling (37) and platelet-derived growth factor signaling (40). Recently, Qin et al. (41) reported findings from metabolome analyses involving nuclear magnetic resonance (NMR)-based and capillary electrophoresis time-of-flight mass spectrometry (CE-TOFMS)-based investigations in ACR-treated human JHH7 HCC cells and normal human hepatic cells (Hc). In this comparison of the metabolic effects of ACR on JHH7 and Hc cells, the authors focused on adenosine-5'-triphosphate (ATP), among a number of metabolites down-regulated upon ACR treatment, and showed an HCC-selective inhibitory effect of ACR on ATP accumulation specifically in JHH7 HCC cells. This effect was associated with ACR-enhanced expression of pyruvate dehydrogenase kinases 4 (PDK4), a key regulator of ATP production. The findings of this study suggest that mitochondrial oxidative phosphorylation may be critical in the energy metabolism of HCC cells. Possible molecular mechanisms through which ACR acts in the prevention of HCC, especially highlighting findings from our research group, are summarized in Figure 1 [modified from reference (42)].
Acyclic retinoid (ACR) for blocking HCC: clinical studies
In a placebo-controlled clinical study, the preventive effects of ACR on second primary HCC were investigated in patients who were free of HCC after surgical resection or percutaneous treatment of the primary liver tumor (43). This study demonstrated that oral administration of ACR for 12 months significantly reduced the incidence of post-therapeutic HCC recurrence. In addition, ACR treatment improved significantly the survival rate of patients (44). Based on these findings, a large-scale randomized double-blind placebo-controlled clinical trial was conducted to confirm the efficacy of ACR against HCC (45). A total of 401 patients, who had curative therapy for HCV-related HCC, were randomized to treatment with oral administration of ACR (600 or 300 mg/day) or placebo for 96 weeks. Upon completion of the study, a total of 377 patients had been followed for the complete study period. The comparison of recurrence-free survival rates between ACR (600 and 300 mg/day) and placebo groups did not show significant difference (P=0.434). The hazard ratios for recurrence-free survival for patients treated with ACR at a dose of 600 mg/day versus placebo group were 0.73 (95% CI, 0.51-1.03) for the overall study period, and 0.27 (95% CI, 0.07-0.96) 2 years after randomization.
Although effectiveness of ACR treatment compared to placebo was not demonstrated in this trial, it was shown that the Child-Pugh liver function classification of the patients affected the observed efficacy of ACR. Upon subgroup analysis, treatment with 600 mg ACR/day had a significant effect in Child-Pugh class A patients, who had less severe liver cirrhosis (hazard ratio, 0.60; 95% CI, 0.41-0.89). In addition, among patients in Child-Pugh class A the overall survival of the 600 mg ACR/day group was significantly longer than that of the placebo group (hazard ratio, 0.575; 95% CI, 0.341-0.967) (46). Since the proportion of Child-Pugh A was approximately 80% in the study participants, another clinical trial now being conducted, focusing on patients with Child-Pugh A disease classification in order to confirm and validate the efficacy of ACR for preventing HCV-related HCC.
Since it is not fully understood how and why ACR shows inhibitory effects on HCC recurrence in humans possible mechanisms for ACR actions have been explored by performing gene expression profiling of liver biopsy samples obtained from patients who had undergone 8-week ACR (600 mg/day) treatment after curative therapy for HCV-related HCC (47). Treatment with ACR elevated expression levels of many retinoid target genes, and decreased primarily tumor progression-related genes. Importantly, HCC recurrence within 2 years could be predicted by this profiling analysis. Of the retinoid-related genes up-regulated by ACR, CCAAT/enhancer-binding protein-α (C/EBP-α) and insulin-like growth factor-binding protein 6 (IGFBP6) have been reported to inhibit cell growth in a hepatoma cell line (48). In addition, the synthetic retinoid bexarotene was found to induce expression of IGFBP6. This synthetic retinoid was reported to suppress cancer cell growth through a process mediated transcriptionally by RAR-β and involving RAR/RXR heterodimer formation (49). Interestingly, in these profiling analyses, C/EBP-α displayed a marked elevation in expression level before and during ACR treatment and this prominent difference in expression levels was able to distinguish between recurrence and non-recurrence groups.
In phase I clinical trial of ACR (50), relatively severe (grade 3, according to the National Cancer Institute Common Toxicity Criteria version 2.0) hypertension was observed in 3 out of 9 enrolled subjects of the 900-mg ACR group, but this treatment-related adverse event was successfully managed by anti-hypertensive drugs. Okita et al. (45) have reported that ACR administration caused several adverse reactions, including onychoclasis, headache, anemia, renal impairment, and edema. The occurrence rate of those events, which caused discontinuation of the trial, increased with ACR dose: 15.9% (21/132) in the 600-mg group, 6.9% (9/131) in the 300-mg group, and 4.7% (6/129) in the placebo group. Other adverse events associated with retinoid treatment, such as mucocutaneous symptoms, impaired lipid metabolism, and musculoskeletal disorders, were rarely observed in the previous study of ACR (45). It can be considered that administration of ACR within clinical dose exhibits no severe and life-threatening complications such as retinoic acid syndrome, a cardiorespiratory syndrome manifested by dyspnea, pulmonary infiltrates, pleural effusions, and so on (51). Clinicians, however, should be careful, because there is a possibility that treatment with ACR for a longer period of time has other unexpected adverse reactions.
Conclusions
The high incidence and recurrence rate of HCC in cirrhotic livers results in a poor prognosis for HCC patients. In order to improve the prognoses for these patients, effective strategies for controlling chronic liver diseases and chemoprevention of HCC must be developed. Findings from both experimental and clinical investigations suggest that treatment with retinoids may offer one of the promising approaches for treating and preventing HCC. In particular, the synthetic retinoid ACR appears to be a promising agent for prevention of HCC development. Its efficacy for preventing HCC recurrence is already validated in subgroup analyses of data from a clinical trial. The mechanisms of ACR action, however, have not yet been fully elucidated and still need further study. Moreover, ACR will possibly be approved for use in HCV-infected patients although not in other patient populations. Further studies examining the beneficial effects of retinoids on liver disease should be increased and expanded into other etiologies, including chronic HBV patients, and patients with alcoholic hepatitis and NAFLD.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Bosch FX, Ribes J, Diaz M, et al. Primary liver cancer: worldwide incidence and trends. Gastroenterology 2004;127:S5-16. [PubMed]
- Bruix J, Boix L, Sala M, et al. Focus on hepatocellular carcinoma. Cancer Cell 2004;5:215-9. [PubMed]
- Shirakami Y, Lee SA, Clugston RD, et al. Hepatic metabolism of retinoids and disease associations. Biochim Biophys Acta 2012;1821:124-36.
- Yanagitani A, Yamada S, Yasui S, et al. Retinoic acid receptor alpha dominant negative form causes steatohepatitis and liver tumors in transgenic mice. Hepatology 2004;40:366-75. [PubMed]
- Hessel S, Eichinger A, Isken A, et al. CMO1 deficiency abolishes vitamin A production from beta-carotene and alters lipid metabolism in mice. J Biol Chem 2007;282:33553-61. [PubMed]
- Yang Q, Graham TE, Mody N, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 2005;436:356-62. [PubMed]
- Balmer JE, Blomhoff R. Gene expression regulation by retinoic acid. J Lipid Res 2002;43:1773-808. [PubMed]
- McGrane MM. Vitamin A regulation of gene expression: molecular mechanism of a prototype gene. J Nutr Biochem 2007;18:497-508. [PubMed]
- Saitou M, Narumiya S, Kakizuka A. Alteration of a single amino acid residue in retinoic acid receptor causes dominant-negative phenotype. J Biol Chem 1994;269:19101-7. [PubMed]
- Koike K. Molecular basis of hepatitis C virus-associated hepatocarcinogenesis: lessons from animal model studies. Clin Gastroenterol Hepatol 2005;3:S132-5. [PubMed]
- Tsuchiya H, Akechi Y, Ikeda R, et al. Suppressive effects of retinoids on iron-induced oxidative stress in the liver. Gastroenterology 2009;136:341-50.e8.
- Ashla AA, Hoshikawa Y, Tsuchiya H, et al. Genetic analysis of expression profile involved in retinoid metabolism in non-alcoholic fatty liver disease. Hepatol Res 2010;40:594-604. [PubMed]
- Inoue M, Kurahashi N, Iwasaki M, et al. Metabolic factors and subsequent risk of hepatocellular carcinoma by hepatitis virus infection status: a large-scale population-based cohort study of Japanese men and women (JPHC Study Cohort II). Cancer Causes Control 2009;20:741-50. [PubMed]
- de Lera AR, Bourguet W, Altucci L, et al. Design of selective nuclear receptor modulators: RAR and RXR as a case study. Nat Rev Drug Discov 2007;6:811-20. [PubMed]
- Kanda S, Nakashima R, Takahashi K, et al. Potent antidiabetic effects of rivoglitazone, a novel peroxisome proliferator-activated receptor-gamma agonist, in obese diabetic rodent models. J Pharmacol Sci 2009;111:155-66. [PubMed]
- Schultz JR, Tu H, Luk A, et al. Role of LXRs in control of lipogenesis. Genes Dev 2000;14:2831-8. [PubMed]
- Shulman AI, Larson C, Mangelsdorf DJ, et al. Structural determinants of allosteric ligand activation in RXR heterodimers. Cell 2004;116:417-29. [PubMed]
- Sherman SI. Etiology, diagnosis, and treatment recommendations for central hypothyroidism associated with bexarotene therapy for cutaneous T-cell lymphoma. Clin Lymphoma 2003;3:249-52. [PubMed]
- Standeven AM, Thacher SM, Yuan YD, et al. Retinoid X receptor agonist elevation of serum triglycerides in rats by potentiation of retinoic acid receptor agonist induction or by action as single agents. Biochem Pharmacol 2001;62:1501-9. [PubMed]
- Kakuta H, Yakushiji N, Shinozaki R, et al. RXR Partial Agonist CBt-PMN Exerts Therapeutic Effects on Type 2 Diabetes without the Side Effects of RXR Full Agonists. ACS Med Chem Lett 2012;3:427-32. [PubMed]
- Tsuchiya H, Ikeda Y, Ebata Y, et al. Retinoids ameliorate insulin resistance in a leptin-dependent manner in mice. Hepatology 2012;56:1319-30. [PubMed]
- Graham TE, Yang Q, Bluher M, et al. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med 2006;354:2552-63. [PubMed]
- Seo JA, Kim NH, Park SY, et al. Serum retinol-binding protein 4 levels are elevated in non-alcoholic fatty liver disease. Clin Endocrinol (Oxf) 2008;68:555-60. [PubMed]
- Klöting N, Graham TE, Berndt J, et al. Serum retinol-binding protein is more highly expressed in visceral than in subcutaneous adipose tissue and is a marker of intra-abdominal fat mass. Cell Metab 2007;6:79-87. [PubMed]
- Xia M, Liu Y, Guo H, et al. Retinol binding protein 4 stimulates hepatic sterol regulatory element-binding protein 1 and increases lipogenesis through the peroxisome proliferator-activated receptor-gamma coactivator 1beta-dependent pathway. Hepatology 2013;58:564-75. [PubMed]
- Lingen MW, Polverini PJ, Bouck NP. Retinoic acid and interferon alpha act synergistically as antiangiogenic and antitumor agents against human head and neck squamous cell carcinoma. Cancer Res 1998;58:5551-8. [PubMed]
- Obora A, Shiratori Y, Okuno M, et al. Synergistic induction of apoptosis by acyclic retinoid and interferon-beta in human hepatocellular carcinoma cells. Hepatology 2002;36:1115-24. [PubMed]
- Hamamoto S, Fukuda R, Ishimura N, et al. 9-cis retinoic acid enhances the antiviral effect of interferon on hepatitis C virus replication through increased expression of type I interferon receptor. J Lab Clin Med 2003;141:58-66. [PubMed]
- Böcher WO, Wallasch C, Hohler T, et al. All-trans retinoic acid for treatment of chronic hepatitis C. Liver Int 2008;28:347-54. [PubMed]
- Bitetto D, Bortolotti N, Falleti E, et al. Vitamin A deficiency is associated with hepatitis C virus chronic infection and with unresponsiveness to interferon-based antiviral therapy. Hepatology 2013;57:925-33. [PubMed]
- Yamada Y, Shidoji Y, Fukutomi Y, et al. Positive and negative regulations of albumin gene expression by retinoids in human hepatoma cell lines. Mol Carcinog 1994;10:151-8. [PubMed]
- Muto Y, Moriwaki H. Antitumor activity of vitamin A and its derivatives. J Natl Cancer Inst 1984;73:1389-93. [PubMed]
- Suzui M, Masuda M, Lim JT, et al. Growth inhibition of human hepatoma cells by acyclic retinoid is associated with induction of p21(CIP1) and inhibition of expression of cyclin D1. Cancer Res 2002;62:3997-4006. [PubMed]
- Adachi S, Okuno M, Matsushima-Nishiwaki R, et al. Phosphorylation of retinoid X receptor suppresses its ubiquitination in human hepatocellular carcinoma. Hepatology 2002;35:332-40. [PubMed]
- Matsushima-Nishiwaki R, Okuno M, Takano Y, et al. Molecular mechanism for growth suppression of human hepatocellular carcinoma cells by acyclic retinoid. Carcinogenesis 2003;24:1353-9. [PubMed]
- Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell 2000;103:211-25. [PubMed]
- Shao RX, Otsuka M, Kato N, et al. Acyclic retinoid inhibits human hepatoma cell growth by suppressing fibroblast growth factor-mediated signaling pathways. Gastroenterology 2005;128:86-95. [PubMed]
- Shimizu M, Suzui M, Deguchi A, et al. Effects of acyclic retinoid on growth, cell cycle control, epidermal growth factor receptor signaling, and gene expression in human squamous cell carcinoma cells. Clin Cancer Res 2004;10:1130-40. [PubMed]
- Nakamura N, Shidoji Y, Moriwaki H, et al. Apoptosis in human hepatoma cell line induced by 4,5-didehydro geranylgeranoic acid (acyclic retinoid) via down-regulation of transforming growth factor-alpha. Biochem Biophys Res Commun 1996;219:100-4. [PubMed]
- Okada H, Honda M, Campbell JS, et al. Acyclic retinoid targets platelet-derived growth factor signaling in the prevention of hepatic fibrosis and hepatocellular carcinoma development. Cancer Res 2012;72:4459-71. [PubMed]
- Qin XY, Wei F, Tanokura M, et al. The effect of acyclic retinoid on the metabolomic profiles of hepatocytes and hepatocellular carcinoma cells. PLoS One 2013;8:e82860. [PubMed]
- Shimizu M, Takai K, Moriwaki H. Strategy and mechanism for the prevention of hepatocellular carcinoma: phosphorylated retinoid X receptor alpha is a critical target for hepatocellular carcinoma chemoprevention. Cancer Sci 2009;100:369-74. [PubMed]
- Muto Y, Moriwaki H, Ninomiya M, et al. Prevention of second primary tumors by an acyclic retinoid, polyprenoic acid, in patients with hepatocellular carcinoma. Hepatoma Prevention Study Group. N Engl J Med 1996;334:1561-7. [PubMed]
- Muto Y, Moriwaki H, Saito A. Prevention of second primary tumors by an acyclic retinoid in patients with hepatocellular carcinoma. N Engl J Med 1999;340:1046-7. [PubMed]
- Okita K, Izumi N, Matsui O, et al. Peretinoin after curative therapy of hepatitis C-related hepatocellular carcinoma: a randomized double-blind placebo-controlled study. J Gastroenterol 2015;50:191-202. [PubMed]
- Okita K, Izumi N, Ikeda K, et al. Survey of survival among patients with hepatitis C virus-related hepatocellular carcinoma treated with peretinoin, an acyclic retinoid, after the completion of a randomized, placebo-controlled trial. J Gastroenterol 2015;50:667-74. [PubMed]
- Honda M, Yamashita T, Arai K, et al. Peretinoin, an acyclic retinoid, improves the hepatic gene signature of chronic hepatitis C following curative therapy of hepatocellular carcinoma. BMC Cancer 2013;13:191. [PubMed]
- Nakanishi M, Tomaru Y, Miura H, et al. Identification of transcriptional regulatory cascades in retinoic acid-induced growth arrest of HepG2 cells. Nucleic Acids Res 2008;36:3443-54. [PubMed]
- Uray IP, Shen Q, Seo HS, et al. Rexinoid-induced expression of IGFBP-6 requires RARbeta-dependent permissive cooperation of retinoid receptors and AP-1. J Biol Chem 2009;284:345-53. [PubMed]
- Okusaka T, Ueno H, Ikeda M, et al. Phase I and pharmacokinetic clinical trial of oral administration of the acyclic retinoid NIK-333. Hepatol Res 2011;41:542-52. [PubMed]
- Larson RS, Tallman MS. Retinoic acid syndrome: manifestations, pathogenesis, and treatment. Best Pract Res Clin Haematol 2003;16:453-61. [PubMed]


