Alcohol exposure in utero perturbs retinoid homeostasis in adult rats
Introduction
Vitamin A is an essential nutrient responsible for regulating and maintaining mammalian vision, embryogenesis, immunity, proliferation and differentiation (1). Once obtained from the diet as retinol, retinyl esters, and pro-vitamin A carotenoids (mainly β-carotene), vitamin A is predominantly stored in the liver as retinyl ester, synthesized by lecithin-retinol acyltransferase (LRAT). From the liver, retinol generated upon hydrolysis of retinyl esters is secreted into the circulation bound to retinol-binding protein (RBP) to reach the extrahepatic target tissues. Within the target cells, retinol dehydrogenases (RDHs), and also alcohol dehydrogenases (ADHs), convert retinol into retinaldehyde, which is subsequently oxidized by retinaldehyde dehydrogenases (RALDHs) to generate retinoic acid, the active form of vitamin A that functions as a transcriptional regulator (2-7).
The well-known detrimental effects of alcohol on adult tissues include the impairment of retinoid (vitamin A and its derivatives) metabolism following both acute and chronic alcohol exposure (8). This effect has been ascribed to the biochemical similarity between ethanol and retinol metabolism, which also share enzymes such as ADH and cytochrome P450 2E1 (CYP2E1) (8). In humans, chronic consumption of alcohol progressively decreases plasma retinol levels and depletes hepatic retinoid stores, in both hepatocytes and stellate cells, affecting both retinol and retinyl ester levels (9-15), and overall, predisposing alcoholics to develop clinical signs of vitamin A deficiency (VAD) (8). Studies in various animal models, although predominantly in rodents, have confirmed the findings in humans with the exception of the decreased plasma retinol levels (8). Interestingly, chronic alcohol consumption also has an effect on extrahepatic retinoid homeostasis by increasing, rather than decreasing, retinoid concentrations in various brain regions, the colon, esophagus, kidney, lung, testes and trachea (16-22). The effects of alcohol consumption on tissue retinoic acid levels have not been unequivocally defined, due to discrepant data reported in the literature (23). Multiple mechanisms have been proposed to explain the effects of alcohol on retinoid metabolism, including the possibility that alcohol stimulates mobilization of hepatic retinoid stores towards extra-hepatic tissues and retinoid catabolism (8).
Retinoid homeostasis in the developing tissues is critical to normal embryonic development in mammals (24). Therefore, both deficiency and excess of vitamin A during gestation result in fetal death or in a spectrum of congenital defects (25). Alcohol exposure in utero has also been shown to perturb retinoid metabolism and signaling in developing tissues (26). Although, an exact mechanism of this disturbance has yet to be established, it has been associated with the development of fetal alcohol spectrum disorders (FASD) (27,28). This term comprises a broad array of diverse pathological conditions of children born from mothers consuming alcohol during pregnancy (29). The most severe of these conditions is called fetal alcohol syndrome (FAS), with central nervous system dysfunctions, impaired growth, facial abnormalities, and both structural and functional brain damage (30). Many features of FAS mimic those of VAD or vitamin A excess, including poor fetal growth, eye and brain deficiency, heart defects, and impaired neural crest cell migration (27). The similarity between VAD and FAS has led to the hypothesis that alcohol-induced embryonic VAD contributes to FAS (27,28). Initially, it was proposed that ethanol competes with retinol for the enzyme ADH IV (ADH-IV), thus reducing the formation of retinoic acid from retinol (28,31). More recently, however, others have postulated that alcohol-induced vitamin A toxicity contributes to FAS, as hippocampal retinoic acid levels were elevated in alcohol-exposed fetuses (21).
In addition to inducing FASD, intrauterine alcohol exposure is also one of many factors known to predispose offspring to adult diseases by deregulating developmental and/or metabolic pathways heavily interconnected to one another. For example, maternal alcohol exposure can predispose progeny to cancer (32-34), circadian disruptions (35-40), and metabolic diseases in response to other stressors in adult life (41). Given that many adult pathological conditions have been attributed to alterations in vitamin A metabolism (42-45), to better understand the mechanisms whereby prenatal exposure to alcohol compromises health later in life, this study investigates the effects of maternal consumption of alcohol on retinoid metabolism of adult offspring in sprague dawley rats. We chose to analyze the liver, lung and prostate as diseases linked to pathological conditions of these organs have been associated with perturbation in retinoid metabolism (42-45). We show that prenatal alcohol exposure in rats affects retinoid metabolism in adult life, in a tissue- and sex-dependent manner.
Materials and methods
Animals and diet
Pregnant Sprague Dawley rats were purchased from Charles River (Wilmington, MA, USA) and individually housed in a controlled environment with a 12-hour light/dark cycle with the period of darkness between the hours of 19:00 and 07:00. Dams were acclimated to the environment for 2 days and, then, fed a liquid diet containing ethanol (alcohol-fed; F1258SP; Bio-Serv, Frenchtown, NJ, USA) or an isocaloric liquid diet (pair-fed; F1259SP; Bio-Serv, Frenchtown, NJ, USA) or an ad libitum rat regular chow (chow-fed; Purina, St Louis, MO, USA; LabDiet 5012). The liquid diets contained 6 IU of vitamin A/mL, and calories from protein, carbohydrates and fat were 15%, 49% and 36%, respectively, giving rise to an estimated calorie intake of about 75 kcal/day and to an estimated vitamin A intake of 450 IU vitamin A/day, based on a consumption of 75 mL diet/day (46). The regular rat chow diet contained 12 IU vitamin A/g and 1.9 ppm of β-carotene/g. Calories from protein, carbohydrates, and fat were 27%, 60% and 13%, respectively, giving rise to an estimated calorie intake of about 70 kcal/day and to an estimated vitamin A intake of 264 IU vitamin A/day, based on a consumption of 20-25 g diet/day (47).
Experimental scheme
Dams were acclimated to the alcohol diet from day 7 to 9 of gestation by feeding a liquid diet containing 2.2% ethanol on day 7 and 4.4% ethanol on day 8. Once acclimated, dams were fed the liquid diet containing 6.7% ethanol from days 9 to 21. This concentration of ethanol represented 35% of total calories/day (32). Dams drinking 6.7% alcohol are expected to present with blood alcohol levels between 100 to 150 mg/dL (48,49), which translates to approximately three to five drinks in 2 hours in women (50). At birth, male and female pups were cross-fostered to dams fed ad libitum with the regular rat chow diet and litters were normalized to 8 pups per dam. Pups were weaned at 21 days of age and fed the regular rat chow diet ad libitum until 60-70 days of age (n=9-13 offspring pups per treatment group) when they were sacrificed by rapid decapitation. Serum, liver, lung and prostate were collected, frozen and stored at −80 °C until further analyses. Prior to freezing, the three lobes of the prostate (dorsal, lateral and ventral) were quickly dissected and stored in separate tubes. All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Rutgers University Institutional Committee on Animal Care.
High-performance liquid chromatography (HPLC)
Measurements of retinol and retinyl ester in serum, and tissue (liver, lung and prostate) were performed by reverse-phase HPLC, as previously described (51,52). Retinol and retinyl esters (retinyl oleate, linoleate, palmitate and stearate) were identified by comparing retention times and spectral data of experimental compounds with those of authentic standards. Retinyl acetate (Sigma, MO, USA) was added as internal standard.
Western blot analysis
Analysis was performed as previously described (4) by using a rabbit polyclonal anti-rat RBP antiserum for immunodetection. Albumin, detected upon treating the membranes with either Coomassie Blue or Ponceau S stain, and detected by a rabbit polyclonal anti-albumin antibody (Abcam, Cambridge, MA, USA), was used as a loading control. The molecular weight of each detected protein is as follow: RBP, 21 kDa and albumin, 65 kDa. The quantification of the membranes was completed by densitometry analysis with Quantity One Program (Bio-Rad, CA, USA).
RNA extraction, cDNA synthesis, and qPCR
RNA extraction, cDNA synthesis, and qPCR were performed as previously described (53,54). To determine changes in gene expression of Lrat, the ∆∆Ct method was used. Gene expression changes were expressed as fold of the control group (PF). List of primers and amplicon size is as follows: LRAT forward primer 5'-AACCGTGTCGCCCATCTAAT-3', LRAT reverse primer 5'-TTCTGAGTGCGTTCCTTGTCA-3', expected amplicon size is 68 bp; 18S reverse primer 5'-GCTGGAATTACCGCGGCT-3', 18S forward primer 5'-CGGCTACCACATCCAAGGAA-3', expected amplicon size is 187 bp.
Statistical analyses
Normality of variables was assessed by the Shapiro-Wilk test. When the data were not normally distributed, values were logarithmically transformed prior to statistical analysis (female serum retinyl esters and hepatic retinol; male liver, lung, ventral and lateral prostate retinyl esters). Normally distributed values were statistically analyzed by one-way ANOVA for the comparison. Not normally distributed values were statistically tested by Kruskal-Wallis followed by Mann-Whitney test for the comparison. A P value of <0.05 was used to establish statistical significance. Analyses were performed by means of SPSS (SPSS Inc., Chicago, IL, USA).
Results
Serum retinoid and RBP levels
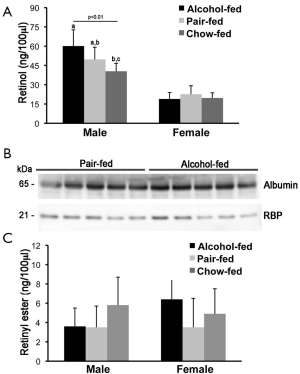
Serum retinol and retinyl ester concentrations were measured by reverse phase HPLC in male and female offspring rats from dams that were alcohol-fed, pair-fed or chow-fed during gestation. As previously reported, serum retinol levels were higher in males than in females rats (55-57). Whereas serum retinol levels were slightly but significantly elevated in the alcohol-fed compared to the chow-fed male group, females rat did not show significant differences in serum retinol concentrations, regardless of the maternal treatment (Figure 1A). In agreement with these data, serum Western blot analysis of RBP and the sole specific retinol carrier in the bloodstream (4), showed similar levels of the protein in both males (Figure 1B) and females (data not shown) when the alcohol-fed and the pair-fed groups were compared. Serum retinyl ester levels were not different among offspring from the three different maternal treatment groups, regardless of the sex (Figure 1C).
Tissue retinol and retinyl ester levels
Retinol and retinyl ester levels were measured by reverse phase HPLC in liver, lung and prostate from the same groups of rats described above. As previously reported, liver retinol and retinyl ester levels were generally higher in female than in male rats (Figure 2A,B) (55-58). Hepatic female retinol levels were significantly lower in the chow-fed group compared to both alcohol-fed and pair-fed female offspring (Figure 2A). In contrast, hepatic retinyl ester levels were not significantly different among female rats from the three different maternal treatment groups (Figure 2B). In the case of the male offspring, whereas hepatic retinol concentrations were similar among the three experimental groups (Figure 2A), hepatic retinyl ester levels were lower in males alcohol-fed vs. pair-fed and chow-fed groups (Figure 2B).
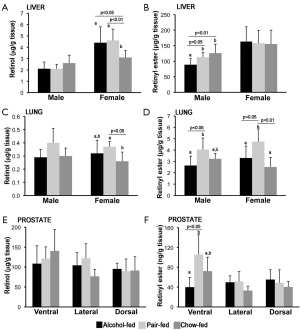
Lung retinol and retinyl ester levels were generally similar in female compared to male rats (55,58). In the male offspring, lung retinol levels where not different among the three experimental groups (Figure 2C), whereas in the females, lung retinol levels were lower in the chow-fed group compared to the pair-fed animals of the same sex (Figure 2C). Retinyl ester levels were significantly lower in alcohol-fed vs. pair-fed, regardless of the gender (Figure 2D). Moreover, lung retinyl ester concentrations were significantly greater in the female pair-fed vs. the chow-fed group of the same sex (Figure 2D).
Finally, in the case of the ventral prostate, whereas retinol levels were unchanged among the various groups (Figure 2E), retinyl ester (Figure 2F) levels were significantly decreased in animals of the alcohol-fed vs. pair-fed group. Retinol and retinyl ester levels were not different in the dorsal and lateral prostate, regardless of the maternal treatment (Figure 2E,F). Note that alterations of the acyl composition of the retinyl ester stores of the offspring from alcohol-fed dams were not observed in any of the tissues analyzed.
Overall, these data indicate that maternal alcohol intake decreased retinyl ester levels in the adult offspring, in a tissue- and sex-dependent manner.
Transcriptional levels of lecithin-retinol acyltransferase (LRAT)
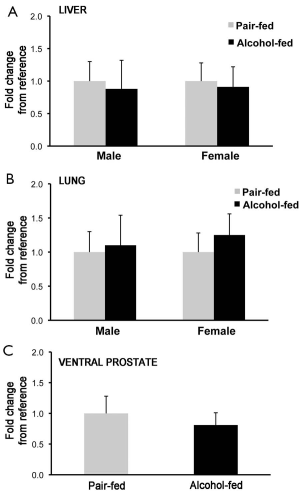
To establish whether the changes in retinyl ester concentrations observed in liver, lung and prostate of the offspring from dams alcohol-fed during pregnancy was correlated to an attenuated transcription of Lrat, the main enzyme that synthesizes retinyl ester in mammalian tissues (2,59), we measured Lrat mRNA levels by qPCR. No differences were observed in Lrat mRNA levels between alcohol-fed and pair-fed groups (males: liver, lung and prostate; females: liver and lung; Figure 3), suggesting that alcohol exposure in utero does not affect Lrat transcription in these organs.
Discussion
Over the past decade, it has become clear that the intrauterine environment plays a critical role in determining the susceptibility to develop chronic pathological conditions, such as diabetes, cardiovascular diseases and cancer, later in life (60). Among the factors that have been shown to influence fetal programming, maternal diet is an important determinant (61).
Alcohol consumption during pregnancy occurs at surprisingly high rates. Roughly 40% of women drink during pregnancy, with about 10% of them drinking heavily (62). Although ideally this condition would be prevented by total sobriety during pregnancy, the prevalence of alcohol use among women of child-bearing age is over 50% (62). Drinking alcohol at any level during pregnancy has been known to cause various pathological conditions of the offspring, depending upon the frequency and dose of alcohol intake, stage of gestation, maternal racial/ethnic population, and nutritional status and/or intake (63-65). In addition to the variety of neurobehavioral and physical abnormalities generically referred to as FASD, intrauterine alcohol exposure has also been linked to a variety of adult diseases of the offspring, likely resulting from the deregulation of developmental and/or metabolic pathways heavily interconnected to one another (66). For example, maternal alcohol consumption has been associated with increased susceptibility to carcinogen-induced mammary (32,33) and prostate (34) tumors in rodents and increased risk of acute myeloid leukemia in children (67), circadian disruption (35-40) and inhibition of insulin signaling in certain tissues (68,69). The mechanisms of such effects are not fully understood. However, it is noteworthy that alcohol has been reported to interfere with the metabolism of various micro- and macro-nutrients such as lipids, vitamin A, folate and choline, for instance. Hence, the hypothesis that some of the effects of maternal (and adult) alcohol intake may be due to dysregulations of the metabolism of such nutrients rather than to a direct effect of alcohol (27,70).
In mammals, retinoids support numerous crucial biological functions (1), and disruption of retinoid homeostasis in tissues has been linked not only to abnormal embryonic development (25), but also to various adult pathological conditions, including proliferative diseases like cancer and skin disease (71-73), metabolic disorders such as obesity, diabetes and dyslipidemia (42-44) and even abnormal lung function (45). Given the crucial role of vitamin A in maintaining the health of the body throughout life, the current study was conducted to investigate whether alcohol exposure in utero may perturb retinoid metabolism in the adult offspring. By using a well-established rat model of prenatal alcohol exposure (34), we observed that maternal alcohol intake affects tissue retinoid concentrations of the adult offspring, in a tissue- and sex-dependent manner. Specifically, the levels of retinyl ester, the storage form of vitamin A, were significantly decreased in the lung of both males and females and in the liver and ventral prostate of males born from dams administered with alcohol during pregnancy. As discussed earlier, the well-known effects of alcohol consumption on both adult and embryonic retinoid metabolism have been linked to the similarity between alcohol and retinol metabolism, even though the molecular details of such interaction still need to be fully clarified (8). Our results add a novel layer of complexity to the relationship between alcohol and vitamin A metabolism suggesting, for the first time, a long-term impact of alcohol on tissue retinoid homesotasis.
Among the multiple mechanisms that have been proposed for the actions of alcohol, it has been shown that alcohol influences gene transcription by inducing various type of epigenetic modifications (74). We therefore hypothesized that the prenatal alcohol exposure in our rat model could have induced epigenetic modifications leading to attenuated transcription of Lrat and, thus, decreased synthesis of retinyl esters. However, the lack of differences in Lrat mRNA levels in the liver, lung and prostate between alcohol-fed and pair-fed groups does not favor this possibility. We cannot exclude, at the moment, that post-transcriptional modifications of the LRAT protein might have possibly occurred resulting in the attenuation of its enzymatic activity, and ultimately in the reduced tissue retinyl ester levels. Alternatively, it could be considered that in utero alcohol exposure might have epigenetically modified genes controlling other key metabolic pathways that maintain tissue retinoid homeostasis, such as hydrolysis of the retinoid stores via retinyl ester hydrolases (75); retinaldehyde oxidation via RALDH2 (6); retinoic acid oxidation into non-active metabolites via CYP26A1 (76); and possibly cellular efflux of retinol mediated by the plasma membrane receptor STRA6 (77-79). All these hypotheses need to be further investigated.
Currently, we do not understand the reasons for the tissue- and sex-specificity of the effect of alcohol on retinoid homeostasis. However, it is interesting that a compromised metabolism of vitamin A in the liver, lung and prostate has been associated with various adult pathological states. Although the cause-effect relationship has not been clarified in all instances, depleted hepatic retinoid stores have been correlated with fatty liver, hepatitis, and cirrhosis in patients with alcoholic liver disease (8). More generally, retinoids control a number of crucial pathways of lipid metabolism that, when compromised, can also lead to non-alcoholic fatty liver disease (NAFLD), which could be considered as the hepatic manifestation of the metabolic syndrome (80). In addition, since retinoids are key regulators of cell proliferation and differentiation (81), a compromised tissue vitamin A homeostasis has been linked to cancer onset and progression at various body sites, including prostate (82) and lung (83). Retinoid metabolism and susceptibility to carcinogenesis have been shown to be different in the various murine prostate lobes (82). Our data confirm that retinoid concentrations seems to be lobe-specific also in the rat prostate, but call for further studies to understand the functional differences that resulted in the retinoid stores being reduced only in the ventral prostate of the offspring from dams alcohol-fed.
Overall, it is intriguing to speculate that adverse influences during development, such as the impairment of tissue retinoid stores induced by maternal alcohol consumption, may cause permanent changes in various organs, leading to increased risk of diseases in adulthood. In our study, we did not measure retinoid levels in fetuses or offspring at birth. Therefore, we cannot establish with certainty that the decline in retinoid stores began in utero. However, it is intriguing that disruption of retinoic acid signaling during embryonic development has been shown to have a negative impact on postnatal lung function in the offspring (45) and that reduced lung function at birth has been associated with an increased risk of airway diseases, such as asthma, later in life (84).
Finally, we would like to point out the sex- and tissue-specific differences in the retinoid concentrations of liver, lung and ventral prostate in the offspring of the dams fed the regular chow diet ad libitum compared to the offspring of the pair-fed dams. At the moment, we do not understand the reason for these differences. It is interesting, however, that the vitamin A intake of the chow-fed and the pair-fed dams was different (264 vs. 450 IU vitamin A/day, respectively), raising the possibility that the intrauterine vitamin A availability per se may also influence the ability to maintain retinoid homeostasis in certain organs later in life, in a sex- and tissue dependent manner.
Conclusions
In summary, this study is the first to report that prenatal alcohol exposure in rats affects retinoid metabolism in adult life, in a tissue- and sex-dependent manner. Given the crucial role of vitamin A in maintaining the health of the body throughout life, these data warrant further investigations to identify the molecular mechanism(s) through which alcohol exposure during embryonic development compromises retinoid metabolism of adult organs such as liver, lung and prostate, likely predisposing to detrimental consequences on health. It is desirable to perform future studies in genetically modified mouse models of altered retinoid metabolism to ease the identification of the targets of the alcohol action.
Acknowledgements
Funding: This work was supported by The Charles and Johanna Busch Memorial Fund and the NJ Agricultural Experiment Station at Rutgers and partially by the National Institute of Health (NIH) grants R01HD057493, R01HD057493-02S1 and R01HD057493-05S1 to Loredana Quadro; and partially by the NIH grant R37AA08757 to Dipak Sarkar.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Rutgers University Institutional Committee on Animal Care.
References
- Sporn MB, Roberts AB, Goodman DS, editors The Retinoids: Biology, Chemistry and Medicine. 2nd ed. New York: Raven Press, 1994.
- O’Byrne SM, Blaner WS. Retinol and retinyl esters: biochemistry and physiology. J Lipid Res 2013;54:1731-43. [PubMed]
- Goodman DW, Huang HS, Shiratori T. tissue distribution and metabolism of newly absorbed vitamin A in the rat. J Lipid Res 1965;6:390-6. [PubMed]
- Quadro L, Blaner WS, Salchow DJ, et al. Impaired retinal function and vitamin A availability in mice lacking retinol-binding protein. EMBO J 1999;18:4633-44. [PubMed]
- Li Y, Wongsiriroj N, Blaner WS. The multifaceted nature of retinoid transport and metabolism. HepatoBiliary Surg Nutr 2014;3:126-39. [PubMed]
- Kedishvili NY. Enzymology of retinoic acid biosynthesis and degradation. J Lipid Res 2013;54:1744-60. [PubMed]
- Al Tanoury Z, Piskunov A, Rochette-Egly C. Vitamin A and retinoid signaling: genomic and nongenomic effects. J Lipid Res 2013;54:1761-75. [PubMed]
- Clugston RD, Blaner WS. The adverse effects of alcohol on vitamin A metabolism. Nutrients 2012;4:356-71. [PubMed]
- Leo MA, Lieber CS. Hepatic vitamin A depletion in alcoholic liver injury. N Engl J Med 1982;307:597-601. [PubMed]
- Majumdar SK, Shaw GK, Thomson AD. Vitamin A utilization status in chronic alcoholic patients. Int J Vitam Nutr Res 1983;53:273-9. [PubMed]
- Smith JC Jr, Brown ED, White SC, et al. Letter: Plasma vitamin A and zinc concentration in patients with alcoholic cirrhosis. Lancet 1975;1:1251-2. [PubMed]
- McClain CJ, Van Thiel DH, Parker S, et al. Alterations in zinc, vitamin A, and retinol-binding protein in chronic alcoholics: a possible mechanism for night blindness and hypogonadism. Alcohol Clin Exp Res 1979;3:135-41. [PubMed]
- Leo MA, Sato M, Lieber CS. Effect of hepatic vitamin A depletion on the liver in humans and rats. Gastroenterology 1983;84:562-72. [PubMed]
- Bell H, Nilsson A, Norum KR, et al. Retinol and retinyl esters in patients with alcoholic liver disease. J Hepatol 1989;8:26-31. [PubMed]
- Adachi S, Moriwaki H, Muto Y, et al. Reduced retinoid content in hepatocellular carcinoma with special reference to alcohol consumption. Hepatology 1991;14:776-80. [PubMed]
- Sato M, Lieber CS. Hepatic vitamin A depletion after chronic ethanol consumption in baboons and rats. J Nutr 1981;111:2015-23. [PubMed]
- Leo MA, Kim C, Lieber CS. Increased vitamin A in esophagus and other extrahepatic tissues after chronic ethanol consumption in the rat. Alcohol Clin Exp Res 1986;10:487-92. [PubMed]
- Mobarhan S, Layden TJ, Friedman H, et al. Depletion of liver and esophageal epithelium vitamin A after chronic moderate ethanol consumption in rats: inverse relation to zinc nutriture. Hepatology 1986;6:615-21. [PubMed]
- Mobarhan S, Seitz HK, Russell RM, et al. Age-related effects of chronic ethanol intake on vitamin A status in Fisher 344 rats. J Nutr 1991;121:510-7. [PubMed]
- Chapman K, Prabhudesai M, Erdman JW Jr. Effects of ethanol and carbon tetrachloride upon vitamin A status of rats. Alcohol Clin Exp Res 1992;16:764-8. [PubMed]
- Kane MA, Folias AE, Wang C, et al. Ethanol elevates physiological all-trans-retinoic acid levels in select loci through altering retinoid metabolism in multiple loci: a potential mechanism of ethanol toxicity. FASEB J 2010;24:823-32. [PubMed]
- Leo MA, Kim CI, Lieber CS. Increased vitamin A in esophagus and lungs after moderate ethanol consumption. Drug Nutr Interact 1988;5:227-36. [PubMed]
- Napoli JL. Effects of ethanol on physiological retinoic acid levels. IUBMB Life 2011;63:701-6. [PubMed]
- Spiegler E, Kim YK, Wassef L, et al. Maternal-fetal transfer and metabolism of vitamin A and its precursor β-carotene in the developing tissues. Biochim Biophys Acta 2012;1821:88-98.
- Clagett-Dame M, Knutson D. Vitamin A in reproduction and development. Nutrients 2011;3:385-428. [PubMed]
- Smith SM. Alcohol-induced cell death in the embryo. Alcohol Health Res World 1997;21:287-97. [PubMed]
- Zachman RD, Grummer MA. The interaction of ethanol and vitamin A as a potential mechanism for the pathogenesis of Fetal Alcohol syndrome. Alcohol Clin Exp Res 1998;22:1544-56. [PubMed]
- Duester G. A hypothetical mechanism for fetal alcohol syndrome involving ethanol inhibition of retinoic acid synthesis at the alcohol dehydrogenase step. Alcohol Clin Exp Res 1991;15:568-72. [PubMed]
- Clarren SK, Smith DW. The fetal alcohol syndrome. N Engl J Med 1978;298:1063-7. [PubMed]
- Haycock PC. Fetal alcohol spectrum disorders: the epigenetic perspective. Biol Reprod 2009;81:607-17. [PubMed]
- Deltour L, Ang HL, Duester G. Ethanol inhibition of retinoic acid synthesis as a potential mechanism for fetal alcohol syndrome. FASEB J 1996;10:1050-7. [PubMed]
- Polanco TA, Crismale-Gann C, Reuhl KR, et al. Fetal alcohol exposure increases mammary tumor susceptibility and alters tumor phenotype in rats. Alcohol Clin Exp Res 2010;34:1879-87. [PubMed]
- Polanco TA, Crismale-Gann C, Cohick WS. Alcohol exposure in utero leads to enhanced prepubertal mammary development and alterations in mammary IGF and estradiol systems. Horm Cancer 2011;2:239-48. [PubMed]
- Murugan S, Zhang C, Mojtahedzadeh S, et al. Alcohol exposure in utero increases susceptibility to prostate tumorigenesis in rat offspring. Alcohol Clin Exp Res 2013;37:1901-9. [PubMed]
- Arjona A, Boyadjieva N, Kuhn P, et al. Fetal ethanol exposure disrupts the daily rhythms of splenic granzyme B, IFN-gamma, and NK cell cytotoxicity in adulthood. Alcohol Clin Exp Res 2006;30:1039-44. [PubMed]
- Arjona A, Boyadjieva N, Sarkar DK. Circadian rhythms of granzyme B, perforin, IFN-gamma, and NK cell cytolytic activity in the spleen: effects of chronic ethanol. J Immunol 2004;172:2811-7. [PubMed]
- Arjona A, Sarkar DK. Circadian oscillations of clock genes, cytolytic factors, and cytokines in rat NK cells. J Immunol 2005;174:7618-24. [PubMed]
- Arjona A, Sarkar DK. The circadian gene mPer2 regulates the daily rhythm of IFN-gamma. J Interferon Cytokine Res 2006;26:645-9. [PubMed]
- Arjona A, Sarkar DK. Evidence supporting a circadian control of natural killer cell function. Brain Behav Immun 2006;20:469-76. [PubMed]
- Arjona A, Sarkar DK. Are circadian rhythms the code of hypothalamic-immune communication? Insights from natural killer cells. Neurochem Res 2008;33:708-18. [PubMed]
- Hellemans KG, Verma P, Yoon E, et al. Prenatal alcohol exposure and chronic mild stress differentially alter depressive- and anxiety-like behaviors in male and female offspring. Alcohol Clin Exp Res 2010;34:633-45. [PubMed]
- Brun PJ, Yang KJ, Lee SA, et al. Retinoids: Potent regulators of metabolism. Biofactors 2013;39:151-63. [PubMed]
- Shirakami Y, Lee SA, Clugston RD, et al. Hepatic metabolism of retinoids and disease associations. Biochim Biophys Acta 2012;1821:124-36.
- Tang XH, Gudas LJ. Retinoids, retinoic acid receptors, and cancer. Annu Rev Pathol 2011;6:345-64. [PubMed]
- Chen F, Marquez H, Kim YK, et al. Prenatal retinoid deficiency leads to airway hyperresponsiveness in adult mice. J Clin Invest 2014;124:801-11. [PubMed]
- Piano MR, Carrigan TM, Schwertz DW. Sex differences in ethanol liquid diet consumption in Sprague-Dawley rats. Alcohol 2005;35:113-8. [PubMed]
- Vento PJ, Swartz ME, Martin LB, et al. Food intake in laboratory rats provided standard and fenbendazole-supplemented diets. J Am Assoc Lab Anim Sci 2008;47:46-50. [PubMed]
- Mihalick SM, Crandall JE, Langlois JC, et al. Prenatal ethanol exposure, generalized learning impairment, and medial prefrontal cortical deficits in rats. Neurotoxicol Teratol 2001;23:453-62. [PubMed]
- Miller MW. Circadian rhythm of cell proliferation in the telencephalic ventricular zone: effect of in utero exposure to ethanol. Brain Res 1992;595:17-24. [PubMed]
- Leeman RF, Heilig M, Cunningham CL, et al. Ethanol consumption: how should we measure it? Achieving consilience between human and animal phenotypes. Addict Biol 2010;15:109-24. [PubMed]
- Kim YK, Quadro L. Reverse-phase high-performance liquid chromatography (HPLC) analysis of retinol and retinyl esters in mouse serum and tissues. Methods Mol Biol 2010;652:263-75. [PubMed]
- Kim YK, Wassef L, Chung S, et al. β-Carotene and its cleavage enzyme β-carotene-15,15'-oxygenase (CMOI) affect retinoid metabolism in developing tissues. FASEB J 2011;25:1641-52. [PubMed]
- Wassef L, Quadro L. Uptake of dietary retinoids at the maternal-fetal barrier: in vivo evidence for the role of lipoprotein lipase and alternative pathways. J Biol Chem 2011;286:32198-207. [PubMed]
- Wassef L, Shete V, Hong A, et al. β-Carotene supplementation decreases placental transcription of LDL receptor-related protein 1 in wild-type mice and stimulates placental β-carotene uptake in marginally vitamin A-deficient mice. J Nutr 2012;142:1456-62. [PubMed]
- Barua AB, McGowan SE, Ivanoff KD, et al. Elevation of retinyl ester level in the lungs of rats following repeated intraperitoneal injections of retinoic acid or retinoyl glucuronide. Pulm Pharmacol Ther 2004;17:113-9. [PubMed]
- Rosales FJ, Jang JT, Piñero DJ, et al. Iron deficiency in young rats alters the distribution of vitamin A between plasma and liver and between hepatic retinol and retinyl esters. J Nutr 1999;129:1223-8. [PubMed]
- Rosales FJ, Ross AC. Acute inflammation induces hyporetinemia and modifies the plasma and tissue response to vitamin A supplementation in marginally vitamin A-deficient rats. J Nutr 1998;128:960-6. [PubMed]
- Ross AC, Li NQ. Retinol combined with retinoic acid increases retinol uptake and esterification in the lungs of young adult rats when delivered by the intramuscular as well as oral routes. J Nutr 2007;137:2371-6. [PubMed]
- O'Byrne SM, Wongsiriroj N, Libien J, et al. Retinoid absorption and storage is impaired in mice lacking lecithin:retinol acyltransferase (LRAT). J Biol Chem 2005;280:35647-57. [PubMed]
- Barker DJ. Sir Richard Doll Lecture. Developmental origins of chronic disease. Public Health 2012;126:185-9. [PubMed]
- Dyer JS, Rosenfeld CR. Metabolic imprinting by prenatal, perinatal, and postnatal overnutrition: a review. Semin Reprod Med 2011;29:266-76. [PubMed]
- Floyd RL, Sidhu JS. Monitoring prenatal alcohol exposure. Am J Med Genet C Semin Med Genet 2004;127C:3-9. [PubMed]
- Abel EL, Hannigan JH. Maternal risk factors in fetal alcohol syndrome: provocative and permissive influences. Neurotoxicol Teratol 1995;17:445-62. [PubMed]
- May PA, Tabachnick BG, Gossage JP, et al. Maternal risk factors predicting child physical characteristics and dysmorphology in fetal alcohol syndrome and partial fetal alcohol syndrome. Drug Alcohol Depend 2011;119:18-27. [PubMed]
- May PA, Gossage JP, Smith M, et al. Population differences in dysmorphic features among children with fetal alcohol spectrum disorders. J Dev Behav Pediatr 2010;31:304-16. [PubMed]
- Zhang X, Sliwowska JH, Weinberg J. Prenatal alcohol exposure and fetal programming: effects on neuroendocrine and immune function. Exp Biol Med (Maywood) 2005;230:376-88. [PubMed]
- Slater ME, Linabery AM, Blair CK, et al. Maternal prenatal cigarette, alcohol and illicit drug use and risk of infant leukaemia: a report from the Children's Oncology Group. Paediatr Perinat Epidemiol 2011;25:559-65. [PubMed]
- de la Monte SM, Wands JR. Role of central nervous system insulin resistance in fetal alcohol spectrum disorders. J Popul Ther Clin Pharmacol 2010;17:e390-404. [PubMed]
- Elton CW, Pennington JS, Lynch SA, et al. Insulin resistance in adult rat offspring associated with maternal dietary fat and alcohol consumption. J Endocrinol 2002;173:63-71. [PubMed]
- Ballard MS, Sun M, Ko J. Vitamin A, folate, and choline as a possible preventive intervention to fetal alcohol syndrome. Med Hypotheses 2012;78:489-93. [PubMed]
- Das BC, Thapa P, Karki R, et al. Retinoic acid signaling pathways in development and diseases. Bioorg Med Chem 2014;22:673-83. [PubMed]
- Alizadeh F, Bolhassani A, Khavari A, et al. Retinoids and their biological effects against cancer. Int Immunopharmacol 2014;18:43-9. [PubMed]
- Duncan FJ, Silva KA, Johnson CJ, et al. Endogenous retinoids in the pathogenesis of alopecia areata. J Invest Dermatol 2013;133:334-43. [PubMed]
- Shukla SD, Velazquez J, French SW, et al. Emerging role of epigenetics in the actions of alcohol. Alcohol Clin Exp Res 2008;32:1525-34. [PubMed]
- Linke T, Dawson H, Harrison EH. Isolation and characterization of a microsomal acid retinyl ester hydrolase. J Biol Chem 2005;280:23287-94. [PubMed]
- Thatcher JE, Isoherranen N. The role of CYP26 enzymes in retinoic acid clearance. Expert Opin Drug Metab Toxicol 2009;5:875-86. [PubMed]
- Isken A, Golczak M, Oberhauser V, et al. RBP4 disrupts vitamin A uptake homeostasis in a STRA6-deficient animal model for Matthew-Wood syndrome. Cell Metab 2008;7:258-68. [PubMed]
- Kim YK, Wassef L, Hamberger L, et al. Retinyl ester formation by lecithin: retinol acyltransferase is a key regulator of retinoid homeostasis in mouse embryogenesis. J Biol Chem 2008;283:5611-21. [PubMed]
- Kawaguchi R, Zhong M, Kassai M, et al. STRA6-catalyzed vitamin A influx, efflux, and exchange. J Membr Biol 2012;245:731-45. [PubMed]
- Sauvant P, Cansell M, Atgié C. Vitamin A and lipid metabolism: relationship between hepatic stellate cells (HSCs) and adipocytes. J Physiol Biochem 2011;67:487-96. [PubMed]
- Gudas LJ, Wagner JA. Retinoids regulate stem cell differentiation. J Cell Physiol 2011;226:322-30. [PubMed]
- Touma SE, Perner S, Rubin MA, et al. Retinoid metabolism and ALDH1A2 (RALDH2) expression are altered in the transgenic adenocarcinoma mouse prostate model. Biochem Pharmacol 2009;78:1127-38. [PubMed]
- Petty WJ, Li N, Biddle A, et al. A novel retinoic acid receptor beta isoform and retinoid resistance in lung carcinogenesis. J Natl Cancer Inst 2005;97:1645-51. [PubMed]
- Checkley W, West KP Jr, Wise RA, et al. Maternal vitamin A supplementation and lung function in offspring. N Engl J Med 2010;362:1784-94. [PubMed]


