Use of irreversible electroporation in unresectable pancreatic cancer
Introduction
Irreversible electroporation is one of the newer novel non-thermal ablative modalities that has been successfully performed intraoperatively (1,2), laparoscopically (3) or percutaneously (4,5). What makes this new palliative option novel is its method of action which does not rely on a thermal-based coagulative necrosis but on a high voltage (maximum 3,000 volts) small microsecond pulse lengths (70 to 90 microseconds). This unique method of action has allowed for IRE to be successfully utilized in locally advanced pancreatic cancer with effective safety and palliation with potentially encouraging improvement in overall survival.
Currently multi-modality therapy including chemotherapy, surgery and/or radiation therapy remains the optimal treatment option for patients with pancreatic adenocarcinoma especially stage II disease. Given the higher incidence of more advanced staged disease (stage III and stage IV), only a small percentage of patients who are diagnosed with pancreatic adenocarcinoma are eligible for definitive surgical resection. Because of this high incidence optimal palliative strategies in order to improve quality-of-life time have become of utmost importance especially in patients with stage III pancreatic adenocarcinoma. The current options for palliation for appropriately and precisely staged locally advanced pancreatic cancer include systemic chemotherapy [Gemcitabine-based or FOLFIRINOX (6)], radiation therapy [IMRT, cyberknife (7) and proton therapy (8)] and surgical therapy [celiac axis alcohol ablation thoracoscopic thoracic splanchnicectomy (9), biliary bypass and gastric bypass]. All of these current modalities have been utilized with various effectiveness and with fairly well-established risks/benefits being known. Currently, optimal quality-of-life parameters have been limited in some of these studies with only the most recent studies demonstrating the stabilization of quality-of-life while undergoing systemic and/or local therapy (10).
Method of action of IRE
Irreversible electroporation in the clinical setting has recently been established to induce permanent cell death through cell membrane perforation which induces electrolyte instability and causes a protracted cell death by apoptosis (11). This immune mediated cell death allows for cellular clearance of this debris and creates a minimal tissue distortion of the surrounding vital structures as have been published in previous clinical follow-up manuscripts. The ability to induce nanopores through effective irreversible electroporation has been demonstrated by electron microscopy in perfused porcine liver (12). Similarly, an optimal dose-response curve has also been validated and established for both the safe use of irreversible electroporation in order to prevent thermal damage as well as the effective use of irreversible electroporation in order to avoid just as importantly reversible electroporation which is synonymous with an ineffective therapy and thus persistence of viable malignancy (13). The tissue effects of irreversible electroporation have also been well established through the ability to irreversibly electroporate the cell membrane alone and to not damage the cartilaginous structures such that vital structures, specifically in locally advanced pancreatic cancer being the superior mesenteric vein (SMV), portal vein complex, the superior mesenteric artery (SMA) and/or celiac order and the bile duct are not thrombosed nor strictured when irreversible electroporation is appropriately performed (2,3,14).
Pre-clinical work and publications
Initial pre-clinical data has been published supporting both the safe and effective use of irreversible electroporation within the pancreas as well as within the hilum of the liver. Bower et al. recently published a chronic animal study demonstrating no adverse events of IRE around the portal venous or SMA complex in a large porcine animal model study. Complete ablations as well as volume ablations were also optimized with this therapy (13). Similar results were confirmed by Charpentier et al. who performed an acute animal model (2 hours survival) and also demonstrated no vascular thrombosis as well as effectiveness of complete ablation (15). Similar studies within the hilum of the liver have also further confirmed the safe and effective use in these non-tumor bearing in vivo porcine models.
Differences of IRE when compared to other thermal injury ablation therapies
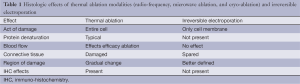
Key to the understanding of the method of action of irreversible electroporation is the understanding of the significance difference when compared to thermal ablative modalities. It has been well established that the method of action on the action of damage, protein denaturation, blood flow, connective tissue, region of damage and the immediacy of the immunohistochemical effects are all significantly different in irreversible electroporation when you compare that to a thermal-based modality (Table 1). It is this difference especially in the ability to pathologically confirm the effects of IRE that has been both its key to method of action but also its significant limitation because of the lack of truly established “treat and resect” type studies. The earliest pathologic confirmation of irreversible electroporation cannot be seen until at least 2 to 4 hours after irreversible electroporation with either electron microscopy or specific immunohistochemical effects as long as the irreversible electroporation tissue has remained perfused for that 2 to 4 hours in order to establish those types of pathologic changes. Additional challenges with IRE has also been around its significant size limitation such that the current optimal size of a locally advanced pancreatic adenocarcinoma should be 3.0 cm or less for the potential new user and 4.0 cm or less for more established IRE end user. The reasons for these are the optimal spacing (1.7 to 2.2) that must be achieved with all probe pairs in order to safely and effectively deliver optimal electrical current between two probes. Inherent to those optimal tumor sizes is also the requirement of appropriate probe pairs being placed with optimal intra-operative image confirmation since variance of greater than 4.0 mm can lead to an ineffective irreversible electroporation (Figure 1). Those probe pairs when placed requires appropriate energy delivery that at times can take upwards to 60 minutes to deliver because of the multiple probe pairs that are required to deliver the energy as well as the optimal probe exposure being no more than 1.0 to 1.5 cm in size which commonly requires at least 2 to 3 pull backs in order to optimal electroporate along the cranial-to-caudal plane. It is this inherent emphasis on the end-user to understand all factors of intra-electroporation energy delivery that is of utmost importance in order to achieve both safety as well as efficacy of the device.
Full table
Current clinical use
The current clinical use of IRE has predominantly been within locally advanced stage III pancreatic adenocarcinoma of either the pancreatic head or body/neck. There have been much smaller percentages of use in margin accentuation for borderline resectable pancreatic tumors, in the treatment of locally recurrent pancreatic adenocarcinoma, as well as within metastatic disease to the pancreas most commonly metastatic renal and melanoma. Key in appropriate patients for the use of IRE is in locally advanced (stage III) only without any evidence of metastatic disease. We commonly utilize at least 4 months of induction chemotherapy in the appropriately and precisely staged stage III pancreatic adenocarcinoma so as to ensure that we are not missing sub radiologically occult microscopic metastatic disease that obviously would not benefit from a local therapy. Appropriately staged locally advanced stage III pancreatic adenocarcinoma at the initial diagnosis must include a high-quality tri-phasic CT scan with thin pancreatic protocol or dynamic MRI in addition to diagnostic laparoscopy and peritoneal washings in order to truly assess and optimally stage and differentiate a stage III pancreatic adenocarcinoma from a potentially sub-radiologically occult stage IV patient. Following that induction chemotherapy repeat staging is then performed to ensure stage III disease is still present and then definitive local therapy and/or additional palliative surgical procedures that being biliary bypass if needed or gastric bypass are needed are performed simultaneously. It is of utmost importance that the appropriate clinician performs irreversible electroporation in locally advanced pancreatic adenocarcinoma. That clinician must have extensive experience in thermal ablative modalities with RFA, microwave and cryoablation as well as being technical facile with the use of high-quality intraoperative procedural imaging most commonly being ultrasound when performed open and/or laparoscopically.
This fairly rigorous staging, induction therapy requirements and high-quality end user understanding and intraoperative imaging has allowed the initial publish experience with the use of IRE in locally advanced pancreatic cancer to be performed safely as well as with encouraging results. Briefly, our initial experience with 27 patients we were able to confirm that IRE of locally advanced pancreatic cancer was both safe and feasible but there were essential keys to safely that being appropriate patient selection, the requirement of high-quality imaging as well as an upper level of understanding in the use of the IRE technology (2). From this initial safety evaluation further comparison of IRE against a group of patients with stage III pancreatic adenocarcinoma who underwent standard-of-care chemotherapy and chemoradiation therapy alone was also performed with initial encouraging results in regards to overall efficacy (1). This report demonstrated an initial hypothesis generating improvement in both local progression-free survival (14 vs. 6 months, P=0.01), improved distant PFS (15 vs. 9 months, P=0.02), as well as improved overall survival (20 vs. 13 months, P=0.03). There have obviously been inherent limitations to the current published results in the use of IRE of pancreatic cancer, the largest being the lack of true understanding as well as true standard-of-care management of patients with locally advanced pancreatic adenocarcinoma. There still remains a wide variability in the use of both induction chemotherapy as well as the timing of utilization of induction radiation therapy in the management of this unique subset of disease. The current largest hurdle that must be overcome in all of the oncology community is a more thorough understanding and acceptance that stage III pancreatic adenocarcinoma is a distinctly different biologic disease than synchronous stage IV metastatic pancreatic adenocarcinoma. Inherent to that acceptance and belief is also the use of high-quality diagnostic imaging and laparoscopy at initial diagnosis. Additional inherent limitations have been to further optimize the quality-of-life improvements that IRE has obtained with an initial signal demonstrating an improvement in overall narcotic use as we have previously published.
Further optimization with the use of IRE in locally advanced pancreatic adenocarcinoma will also come from standardization of technique in regards to optimal probe placement which we believe must be performed in a transmesocolic caudal-to-cranial needle insertion with continuous intraoperative ultrasound imaging being utilized from needle insertion to needle endpoint in order to avoid any type of underlying needle damage to vital structures. Optimal probe exposure being 1.0 to 1.5 cm at maximum as well as understanding of clinical irreversible electroporation endpoints with initial signal demonstrating that an overall change in resistance is going to be more optimally reproducible than any type of intra-ablation ultrasound imaging assessment because of the significant amount of edema that occurs with and after IRE delivery.
In conclusion IRE of locally advanced pancreatic adenocarcinoma is not a standard-of-care practice this time because of a number of keys to acceptance. First and foremost must be an overall optimization in staging and diagnosis of locally advanced pancreatic adenocarcinoma and the paradigm shift to stop grouping this patient with known stage IV metastatic disease. Additional keys will also be standardization of needle device placement as well as optimization of intra-electroporation efficacy endpoints, which are currently being optimized. After those keys have been established then a true validation either single-arm or randomized phase II study will have to be performed in order to truly validate the utilization of IRE in locally advanced pancreatic adenocarcinoma as an optimal treatment in patients who have undergone appropriate induction chemotherapy after they have been appropriately staged.
Acknowledgements
Disclosure: RC Martin II is a paid consultant for Angiodynamics.
References
- Martin RC 2nd, McFarland K, Ellis S, et al. Irreversible electroporation in locally advanced pancreatic cancer: potential improved overall survival. Ann Surg Oncol 2013;20 Suppl 3:S443-9. [PubMed]
- Martin RC 2nd, McFarland K, Ellis S, et al. Irreversible electroporation therapy in the management of locally advanced pancreatic adenocarcinoma. J Am Coll Surg 2012;215:361-9. [PubMed]
- Cannon R, Ellis S, Hayes D, et al. Safety and early efficacy of irreversible electroporation for hepatic tumors in proximity to vital structures. J Surg Oncol 2013;107:544-9. [PubMed]
- Bagla S, Papadouris D. Percutaneous irreversible electroporation of surgically unresectable pancreatic cancer: a case report. J Vasc Interv Radiol 2012;23:142-5. [PubMed]
- Narayanan G, Hosein PJ, Arora G, et al. Percutaneous irreversible electroporation for downstaging and control of unresectable pancreatic adenocarcinoma. J Vasc Interv Radiol 2012;23:1613-21. [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [PubMed]
- Shen ZT, Wu XH, Li B, et al. Preliminary efficacy of CyberKnife radiosurgery for locally advanced pancreatic cancer. Chin J Cancer 2010;29:802-9. [PubMed]
- Hsiung-Stripp DC, McDonough J, Masters HM, et al. Comparative treatment planning between proton and X-ray therapy in pancreatic cancer. Med Dosim 2001;26:255-9. [PubMed]
- Reddy SK, Burton AW. Re: video-assisted thoracoscopic sympathectomy-splanchnicectomy. J Pain Symptom Manage 2002;23:177. [PubMed]
- Gourgou-Bourgade S, Bascoul-Mollevi C, Desseigne F, et al. Impact of FOLFIRINOX compared with gemcitabine on quality of life in patients with metastatic pancreatic cancer: results from the PRODIGE 4/ACCORD 11 randomized trial. J Clin Oncol 2013;31:23-9. [PubMed]
- Lee EW, Loh CT, Kee ST. Imaging guided percutaneous irreversible electroporation: ultrasound and immunohistological correlation. Technol Cancer Res Treat 2007;6:287-94. [PubMed]
- Lee EW, Wong D, Prikhodko SV, et al. Electron microscopic demonstration and evaluation of irreversible electroporation-induced nanopores on hepatocyte membranes. J Vasc Interv Radiol 2012;23:107-13. [PubMed]
- Bower M, Sherwood L, Li Y, et al. Irreversible electroporation of the pancreas: definitive local therapy without systemic effects. J Surg Oncol 2011;104:22-8. [PubMed]
- Maor E, Ivorra A, Leor J, et al. The effect of irreversible electroporation on blood vessels. Technol Cancer Res Treat 2007;6:307-12. [PubMed]
- Charpentier KP, Wolf F, Noble L, et al. Irreversible electroporation of the pancreas in swine: a pilot study. HPB (Oxford) 2010;12:348-51. [PubMed]


