Role of liver progenitors in liver regeneration
Introduction
Parenchymal liver injury and the subsequent cellular response is strongly associated with the etiology and severity of injury. Mature hepatocytes represent the major cell type within the liver. Hepatocytes are unipotent cells contributing to normal cell turnover and are able to respond rapidly to injurious stimuli. In contrast, the liver progenitor cell (LPC) compartment only expands when the regenerative capacity of hepatocytes is continually and severely compromised, a feature common to most chronic liver diseases (1,2). When cell death exceeds the self regenerative capacity of the liver, liver failure occurs and orthotopic liver transplantation (OLT) becomes necessary. OLT still represents the only potentially curative treatment option for several acute, chronic inborn or acquired liver diseases. Donor organ shortage (3) and mandatory life long immunosuppression (4) have prompted investigators to search for alternative treatment options. To date, the only therapeutic alternative to OLT which has reached clinical application is transplantation of mature hepatocytes in individuals with live threatening hepatic diseases (where organ transplantation would remain the only treatment option). Ideal candidates for this therapy are patients with acute liver failure (ALF) (5,6) and liver inborn defects of metabolism (7). Hepatocyte transplantation is less invasive as hepatocytes are delivered through the vascular system (i.e., intraportal route), which may be less harmful for critically ill patients. However, this method is limited to individuals whose liver architecture remains preserved, since it is required for cell engraftment and expansion. Hepatocyte transplantation in patients with advanced fibrosis or cirrhosis is hampered by difficulties in engraftment and risk of portal hypertension (8). Furthermore, it remains unclear if hepatocyte transplantation can achieve a sustained improvement of liver function, since the number of hepatocytes required to improve liver function far exceeds the number of cells that can be safely administered (usually less than 1% of the liver mass). A major limitation to hepatocyte transplantation is the lack of hepatocytes of sufficient quality. For these reasons, investigators have turned to alternative stem cell based therapies, since one of the promises of stem cells is their potential to provide a renewable source of hepatocytes (9).
This review aims to provide a concise overview on: (I) the role of resident LPCs during liver reconstitution and signalling pathways involved during regeneration; (II) the implication of pluripotent embryonic stem (ES) cells and differentiated stem/progenitor cells as potential transplantable assistance for liver regeneration.
Liver reconstitution by resident LPCs in the niche
During the early stages of liver damage, inflammatory cytokines trigger hepatocytes to enter the cell cycle. In moderate liver damage, differentiated hepatocytes can replace neighbouring cells within few days. The LPC compartment is only activated when insufficient numbers of healthy hepatocytes remain to perform regenerative processes (see Figure 1), i.e., in most chronic liver diseases as well as ALF. Katoonizadeh et al. suggested, that at least 50% hepatocyte loss and hepatocyte replicative senescence are necessary to trigger LPC activation (10). The bipotential LPCs, in rodents historically termed “oval cells”, reside in the Canals of Herring (CoH), located in the niche of the biliary-hepatocytic interface (11). In human liver, activation of LPC compartment is referred to as “Ductular Reaction” (DR) due to the more ductular proliferation phenotype that arises during LPC expansion. LPCs are able to infiltrate along the liver plate towards the central vein and differentiate into hepatocytes as well as cholangiocytes. In the niche, LPCs are surrounded by epithelial cells, non-parenchymal cells (i.e., hepatic stellate cells, HSCs), as well as immune cells and extracellular matrix (ECM). Thus, activating signals from various sources can easily reach the LPCs. The activation and expansion of the LPC compartment occurs roughly over 7 days, while the process of LPC differentiation into intermediate hepatocytes requires an additional 7 days (12,13). Thus, the LPC response is a much slower regenerative process compared to hepatocyte replication.
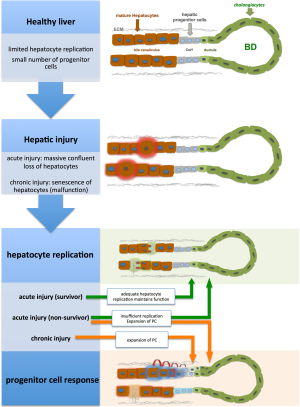
LPC activation has been described in various hepatic disease conditions such as acute liver necrosis (14,15), hemochromatosis (16), chronic cholestatic diseases (14), alcoholic liver disease (17) and chronic viral hepatitis (18). In contrast, conditions of extrahepatic biliary obstruction reveal typical ductular reactions, which show proliferation of mature cholangiocytes only, but not of LPCs (19). LPC activation is also absent or minimal during liver regeneration after partial hepatectomy (PH) to an extent of up to 2/3 of rat liver (20). In consistency with the findings mentioned above, Lowes and colleagues could furthermore describe a positive correlation between severity of hemochromatosis, alcoholic liver disease, chronic hepatitis C and increase in the number of oval cells. In addition, this observation is consistent with the hypothesis that oval cell proliferation is associated with increased risk for development of hepatocellular carcinoma during chronic liver disease (16).
Cell signalling axes regulating LPC mediated regeneration
The regenerative process upon liver injury is orchestrated by a complex cross-talk between different liver cell compartments, and is mediated by cytokines, mitogens and several growth factors (see Figure 1) (21). Chronic liver disease is characterized by apoptosis, necrosis and senescence of hepatocytes (22) and is associated with activation and expansion of resident LPCs (23,24). Cytokines including Interleukine 6 (IL6), tumor necrosis factor alpha (TNF alpha), Osteopontin, transforming growth factor beta (TGF beta), transcription factors nuclear factor kappa B (NF-κB) and CCAAT enhancer binding protein beta, as well as growth factors hepatocyte growth factor (HGF) and epidermal growth factor (EGF) (25) are putative factors which promote LPC-mediated regeneration. Additionally, hormones (e.g., Somatostatin, Insulin) (26), adipokines (adipose tissue derived cytokines) (27,28), and neurotransmitters (serotonin, epinephrine, norepinephrine) also regulate LPC function and stimulate the proliferation and differentiation of LPCs to mature hepatocytes and cholangiocytes. There is increasing evidence that morphogenic signals (factors regulating embryonic development) orchestrate the LPC response.
The following axes have been previously described to play a pivotal role in modulation of LPC mediated liver tissue reconstitution:
- TWEAK—Fn14: LPC response is initialised by the TNF-like weak apoptosis inducing factor (TWEAK)/Fibroblast inducible 14 (Fn14) pathway (29). Signalling through this pathway selectively promotes LPC expansion, but does not affect viability of mature hepatocytes during chronic liver injury (30-32). Using Fn14-deficient mice, Karaca and colleagues proposed that the TWEAK/Fn14 axis could directly stimulate LPC expansion in a setting of acute liver injury (33). Bird and coworkers also demonstrated that a single bone marrow cell (BMC) infusion could directly activate a DR. Interestingly, they could show that the macrophage subset within BMC was necessary for the DR and the expansion of clonogenic LPCs. Furthermore, macrophage-derived TWEAK/Fn14 signalling was the key driver of the DR since recombinant TWEAK administration resulted in a similar expansion of LPC in vivo. TWEAK then signals via the TWEAK receptor Fn14 and downstream NF-κB to induce LPC proliferation (34). Another known source for TWEAK are CXCR4+ T cells, which can be recruited by chemoattractant cytokines (i.e., SDF-1). Thus, TWEAK signalling might be a promising target for future LPC mediated therapy trials, i.e., by agonists activating Fn14 (35);
- Hedgehog (Hh) signalling: recent studies showed that Hh pathway activation occurs during liver regeneration after PH (32,36-38). The Hh pathway usually orchestrates fetal tissue and organ development, and stimulates the expansion and viability of stem cells (39. In a rodent study performed by Ochoa and coworkers a 70% PH (a model of liver regeneration) was followed by expansion of liver progenitors. These progenitor cells were Hh-responsive and secreted Hh-ligands. Interestingly, inhibiting the Hh pathway with cyclopamine attenuated the LPC response, repressed expression of progenitor markers, and reduced overall survival (32). Hh ligands over-expressed during liver injury could directly stimulate LPCs to secrete chemokines that lead to the additional recruitment of cells orchestrating regeneration (40). In addition to a direct effect on LPCs, Hh ligands could also enhance LPC proliferation indirectly via activation of HSCs into matrix-producing myofibroblasts (41). Grzelak et al. could demonstrate in a mouse model of thioacetamide (TAA), representative of chronic liver injury, that injured hepatocytes produce Hh ligands. As described above these ligands induced expansion of the LPC population via primary cilia, cellular structures which are crucially required to transduce the Smoothened-dependent Hh signal in vivo (42,43). Taken together the Hh pathway seems to be crucial for involvement of LPCs for liver regeneration;
- Thyroid hormone signalling: extra-hepatic signals influencing liver regeneration are of major interest to facilitate regeneration (44,45). The thyroid hormone T3 modulates cell growth, differentiation, and metabolic functions via interaction with thyroid hormone nuclear receptors (TRs). In an experimental rodent model of 70% and 90% hepatectomy, administration of exogenous T3 significantly enhanced liver regeneration (increased liver body weight ratio and Ki67 index) (46). The underlying mechanisms by which T3 mediates these regenerative effects are unclear, but may be due to upregulation of cyclin D1 and subsequent cell cycle entry (44,45). T3 may also directly induce LPC activation. In a rodent model of combined 2-acetylaminofluorene feeding and PH, László et al. reported that T3 administration enhanced LPC differentiation into hepatocytes (47). Future studies are needed to dissect the downstream signals by which T3 modulates LPC function;
- Fibroblast growth factor 7 (FGF7): HSCs are known to interact with LPCs physically and by paracrine signals (48). During liver injury, Thy1+ mesenchymal cells (Thy1+ MC) were found to expand in the periportal region along with, and in close proximity to, LPCs (49). Expansion of Thy1+ MCs in the periportal region secreting FGF7 stimulated LPC activation upon liver injury (50). On LPCs expression of the FGF7 cognate receptor FGFR2b was confirmed. In a mouse model Takase and colleagues confirmed, that FGF7-deficient mice exhibited markedly depressed LPC expansion upon toxin-induced liver injury (51). These findings provide evidence that FGF7 might play a role as LPC regulator and thus might be a potential therapeutic target for liver diseases;
- The β-catenin/Wnt pathway: β-catenin is implicated in the regulation of liver development, homeostasis, metabolism, regeneration, and carcinogenesis. It plays a pivotal role in stem cell maintenance and renewal, proliferation, differentiation, cell migration, and polarity. In the canonical Wnt signalling pathway, β-catenin is the central component that mediates the Wnt signalling from the cell membrane to the cytoplasm (52). Upon canonical Wnt signalling activation, free β-catenin translocates to the nucleus and binds to TCF/LEF transcription factors, inducing transcriptional activation of target genes, such as NF-κB, resulting in release of pro-inflammatory cytokines (53). Numerous studies in animal models implicate, that β-catenin signalling plays a role in progenitor cell induction and proliferation. Hu and coworkers could demonstrate that DDC diet induced Wnt signalling parallel to significantly increased progenitor cell activation in mice (54). Since the contribution of Wnt signalling to hepatocarcinogenesis is still unclear, clinical trials of Wnt pathway modulation to ameliorate liver disease should be considered with caution;
- HIPPO—YAP pathway: the transcriptional regulators YAP and TAZ as effectors of the HIPPO signalling cascade are the focus of intense interest due to their remarkable biological properties in development, tissue homeostasis and cancer. The Hippo/YAP-signalling pathway seems to play a crucial role in regulation of the liver size (55,56). Manipulation of HIPPO pathway activity leads to profound changes in liver cell proliferation. YAP overexpression results in approximately a 4-fold increase in liver size within weeks (55,56). The precise mechanism of YAP/TAZ-dependent regulation of cell proliferation remains unclear. It is postulated, that the interaction with TEAD cofactors might be essential for promotion of cell growth in some contexts.
LPC activation and expansion parallels fibrogenesis
As described above the LPC niche consists of epithelial (hepatocytes and cholangiocytes) cells, HSCs, and immune cells (i.e., Kupffer cells) interwoven with the ECM. Cumulative evidence shows that the LPC compartment is intricately linked to the ECM (57-59). For example, laminin-LPC interactions are crucial for LPC-mediated parenchymal reconstitution, and the inhibition of ECM remodelling impairs LPC activation and expansion (60). Moreover, direct interaction of LPCs and HSCs has been demonstrated via lymphotoxin beta (LTb) (61). LPCs seem to produce LTb in response to TNF signalling (62) to recruit other LPCs, HSCs, and leukocytes to sites of injury. Blocking of the LTb receptor on HSCs abrogated the fibrotic response to dietary induced liver damage in a mouse model (61). Another mediator of HSC activation by LPCs might be the above described TWEAK. Macrophages and NK cells comprise the main producers of TWEAK, which induces proliferation of LPCs (30). In various mouse models of liver damage knockout of Fn14 (the TWEAK receptor) or antibody treatment against TWEAK blocked the fibrogenic response associated with LPC expansion (63,64). Though, it is not clear if TWEAK itself stimulates HSC activation via Fn14 or if LPCs directly activate HSCs by LTb secretion. These findings demonstrate that the LPC response (DR) occurs parallel to a fibrogenic response (65). Thus, short term accumulation of collagen matrix might be a beneficial prerequisite for physiological repair (which precedes parenchymal cell reconstitution). In analogy to the findings in mouse models, the fibrous tissue scaffold is the LPC niche that facilitates LPC activation and differentiation. To further characterize how ECM composition modulates the LPC response during liver regeneration, future studies investigating the effects of modifying ECM components and the resulting effects on LPC activation, proliferation, differentiation and expansion are required.
Transplantation of stem cells as potential source of liver regeneration
Stem cells and their descendants (progenitor cells) are capable of sustained proliferation and differentiation into specialized cells (66). Stem cells are defined by their ability to self-renew to maintain a cell population with identical properties by symmetric and asymmetric cell division (67).
Stem cell based therapies might be applied in conditions of ALF (68), inborn errors of metabolism, autoimmune or viral hepatitis, toxic injury or (non) alcoholic liver disease (69). Common to all cell transplantation based therapies is the need of a well preserved liver architecture as prerequisite, facilitating regeneration. Stem cells can broadly be divided into pluripotent ES cells and multipotent, adult/differentiated stem/progenitor cells (see Figure 2), the latter can be found in various fetal and postnatal tissues (e.g., bone marrow, adipose tissue, blood). Due to combined abilities of unlimited expansion potential and pluripotency, ES appear to be an ideal source for tissue replacement after injury or in metabolic disease. However, there are currently no approved treatments utilizing ES for several reasons: the accumulation of spontaneous mutations and chromosomal rearrangements degrades their practical utility (70). Furthermore, ethical issues and technical hurdles, such as differentiating ES cells into usable cells while avoiding transplant rejection are current problems preventing a broad medical implication at present.
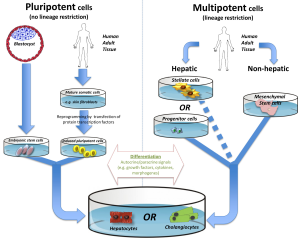
Another subgroup of pluripotent stem cells, so called induced pluripotent stem cells (iPS), can be generated by reprogramming mature somatic cells (71,72) (e.g., derived from human adult skin tissue) by retroviral transfection of various transcription factors. In theory, autologous administration of iPS should not even require immunosuppression (73). At present, broad clinical application is hampered by the same hurdles as described for ES.
In contrast, determined/adult stem cells are transiently amplifying cells during development or regeneration and restricted to one lineage. In the liver adult stem cells are represented by LPCs, which can be easily isolated and cultured (74-76). Effective use was demonstrated by transplantation via intravascular injection into the liver (76,77). In the past, the major limiting factor in the study of LPCs has been the inability to identify, isolate or purify these cells in a reliable fashion. Recently, Cardinale and colleagues successfully isolated multipotent stem/progenitor cells from the human biliary tree by extended cell culture techniques (78) and demonstrated that these progenitor cells are capable of giving rise to hepatocytes, cholangiocytes, and pancreatic islets. Similarly a LPC isolation protocol has been developed for mouse and human liver tissue, but using fluorescence-activated cell sorting (FACS), based on the observation that progenitor cells express high levels of aldehyde dehydrogenase (ALDH) activity. FACS-ALDH1 positive LPCs in culture could give rise to functional hepatocyte-like cells as illustrated by albumin and urea secretion and cytochrome P450 activity (74). These and other novel methods of LPC isolation could well pave the way for the development of future therapies.
Therapeutic effects of mesenchymal stem cells (MSCs) in liver failure are based on their ability to differentiate into hepatocytes and to alter function of immune cells responsible for acute liver injury. They release trophic and immunomodulatory factors (74,79,80) and attenuate proliferation of NKT cells and promote apoptosis of stellate cells. MSCs are assigned to the adult stem cell population and can be found in the perivascular compartment of the liver and most other organs (81,82). They can be isolated from a variety of tissues, such as bone marrow (83), adipose tissue (84), umbilical cord tissue (85) and amniotic fluid (86,87) and can give rise to hepatocyte like cells. MSCs can be expanded ex vivo for multiple passages, but not indefinitely (88). As a promising source for transplantation to cure several hepatic disorders, due to their low MHC I and absence of MHC II expression, MSCs seem to have a low rejection risk upon their administration (89). Apparently MSCs play an increasing role as a source of transplantable cells in several types of liver disease. There are multiple clinical trials either completed, actively recruiting or enrolling patients suffering from cirrhosis (mainly alcoholic) but also cases of ALF, which are treated with MSC transplantation (see Figure S1). The majority of clinical trials conducted on MSCs for the treatment of liver diseases are based on three different sources of cells for transplantation: (I) unfractionated bone marrow, peripheral blood or cytokines (e.g., G-CSF) to mobilize cells; (II) immunoselected cell populations (CD34+ cells, CD133+ cells) from bone marrow or peripheral blood; (III) cultured MSCs or cultures treated with growth factors (e.g., HGF, EGF, fibroblast growth factor) (90).
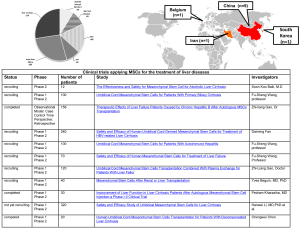
Besides cytokines and growth factors there are also several chemical compounds (dexamethasone, etc.), transcriptional factors (hepatocyte nuclear factor 3β), or cell types (the human hepatoma cell line Huh7, fetal liver cells, HSCs, etc.) promoting differentiation and maturation of MSCs towards hepatocytes (91). Although multiple approaches to this method are available now, all procedures are still lacking further validation. Thus, MSC based therapy remains a challenging issue requiring close cooperation between researchers and clinicians.
Conclusions
OLT as treatment for acute and terminal chronic liver diseases depends on sufficient donor organs and requires long-term immunosuppression. Additional alternative therapeutic options like hepatocyte transplantation have emerged and are warranted. However, the potential of this latter technique has been constrained by numerous technical hurdles. Recent approaches aim to harness stem cell based alternatives, such as induction of resident liver progenitor cells, which could possibly be facilitated by ECM associated factors in the future. Another option is transplantation of non-liver stem cells. Pluripotent cells (ES, iPS) carry the risk of spontaneous mutations and chromosomal rearrangements. Transiently amplifying LPCs have shown great potential in vitro and in vivo, thus first clinical trials might be launched in the coming few years. At present, MSCs represent the most promising source of stem cells with the highest number of ongoing clinical trials. They can potentially alleviate disease conditions by immunomodulatory as well as paracrine signalling mechanisms for months to years.
Before stem cells can be utilized for liver repopulation in humans, still several problems have to be solved, such as the lack of consensus about the immune phenotype of LPCs, obtaining a sufficient quantity of cells for the desired clinical application, ethical aspects, and long term efficacy and safety (92).
Acknowledgements
Funding: Polkemmet Trust and CORE (Wing-Kin Syn); Belgian Federal Science Policy Office (Interuniversity Attraction Poles program—P6/20 and P7/83-HEPRO) and the Brussels Capital Region (INNOVIRIS Impulseprogramme-LifeSciences 2007 and 2011; BruStem) (Jan Best, Laurent Dollé and Leo A. van Grunsven); German Research Foundation (DFG CA267/11-1 and CA267/13-1 to Ali Canbay).
Disclosure: The authors declare no conflict of interest.
References
- Santoni-Rugiu E, Jelnes P, Thorgeirsson SS, et al. Progenitor cells in liver regeneration: molecular responses controlling their activation and expansion. APMIS 2005;113:876-902. [PubMed]
- Dollé L, Best J, Mei J, et al. The quest for liver progenitor cells: a practical point of view. J Hepatol 2010;52:117-29. [PubMed]
- Biancofiore G, Davis CL. Renal dysfunction in the perioperative liver transplant period. Curr Opin Organ Transplant 2008;13:291-7. [PubMed]
- Mukherjee S, Botha JF, Mukherjee U. Immunosuppression in liver transplantation. Curr Drug Targets 2009;10:557-74. [PubMed]
- Pareja E, Gomez-Lechon MJ, Cortes M, et al. Human hepatocyte transplantation in patients with hepatic failure awaiting a graft. Eur Surg Res 2013;50:273-81. [PubMed]
- Wang F, Zhou L, Ma X, et al. Monitoring of intrasplenic hepatocyte transplantation for acute-on-chronic liver failure: a prospective five-year follow-up study. Transplant Proc 2014;46:192-8. [PubMed]
- Mazariegos G, Shneider B, Burton B, et al. Liver transplantation for pediatric metabolic disease. Mol Genet Metab 2014;111:418-27. [PubMed]
- Timm F, Vollmar B. Heterogeneity of the intrahepatic portal venous blood flow: impact on hepatocyte transplantation. Microvasc Res 2013;86:34-41. [PubMed]
- Stutchfield BM, Forbes SJ, Wigmore SJ. Prospects for stem cell transplantation in the treatment of hepatic disease. Liver Transpl 2010;16:827-36. [PubMed]
- Katoonizadeh A, Nevens F, Verslype C, et al. Liver regeneration in acute severe liver impairment: a clinicopathological correlation study. Liver Int 2006;26:1225-33. [PubMed]
- Saxena R, Theise N. Canals of Hering: recent insights and current knowledge. Semin Liver Dis 2004;24:43-8. [PubMed]
- Fausto N. Liver regeneration and repair: hepatocytes, progenitor cells, and stem cells. Hepatology 2004;39:1477-87. [PubMed]
- Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology 2006;43:S45-53. [PubMed]
- Roskams T, De Vos R, Van Eyken P, et al. Hepatic OV-6 expression in human liver disease and rat experiments: evidence for hepatic progenitor cells in man. J Hepatol 1998;29:455-63. [PubMed]
- Fujita M, Furukawa H, Hattori M, et al. Sequential observation of liver cell regeneration after massive hepatic necrosis in auxiliary partial orthotopic liver transplantation. Mod Pathol 2000;13:152-7. [PubMed]
- Lowes KN, Brennan BA, Yeoh GC, et al. Oval cell numbers in human chronic liver diseases are directly related to disease severity. Am J Pathol 1999;154:537-41. [PubMed]
- Ray MB, Mendenhall CL, French SW, et al. Bile duct changes in alcoholic liver disease. The Veterans Administration Cooperative Study Group. Liver 1993;13:36-45. [PubMed]
- Libbrecht L, Desmet V, Van Damme B, et al. Deep intralobular extension of human hepatic ‘progenitor cells’ correlates with parenchymal inflammation in chronic viral hepatitis: can ‘progenitor cells’ migrate? J Pathol 2000;192:373-8. [PubMed]
- Desmet V, Roskams T, Van Eyken P. Ductular reaction in the liver. Pathol Res Pract 1995;191:513-24. [PubMed]
- Fausto N. Liver regeneration: from laboratory to clinic. Liver Transpl 2001;7:835-44. [PubMed]
- Parola M, Pinzani M. Hepatic wound repair. Fibrogenesis Tissue Repair 2009;2:4. [PubMed]
- Ghavami S, Hashemi M, Kadkhoda K, et al. Apoptosis in liver diseases--detection and therapeutic applications. Med Sci Monit 2005;11:RA337-45. [PubMed]
- Duncan AW, Dorrell C, Grompe M. Stem cells and liver regeneration. Gastroenterology 2009;137:466-81. [PubMed]
- Fellous TG, Islam S, Tadrous PJ, et al. Locating the stem cell niche and tracing hepatocyte lineages in human liver. Hepatology 2009;49:1655-63. [PubMed]
- Campbell JS, Prichard L, Schaper F, et al. Expression of suppressors of cytokine signaling during liver regeneration. J Clin Invest 2001;107:1285-92. [PubMed]
- Jung Y, Oh SH, Witek RP, et al. Somatostatin stimulates the migration of hepatic oval cells in the injured rat liver. Liver Int 2012;32:312-20. [PubMed]
- Diehl AM. Lessons from animal models of NASH. Hepatol Res 2005;33:138-44. [PubMed]
- Nobili V, Carpino G, Alisi A, et al. Hepatic progenitor cells activation, fibrosis, and adipokines production in pediatric nonalcoholic fatty liver disease. Hepatology 2012;56:2142-53. [PubMed]
- Alison MR, Islam S, Lim S. Stem cells in liver regeneration, fibrosis and cancer: the good, the bad and the ugly. J Pathol 2009;217:282-98. [PubMed]
- Jakubowski A, Ambrose C, Parr M, et al. TWEAK induces liver progenitor cell proliferation. J Clin Invest 2005;115:2330-40. [PubMed]
- Tirnitz-Parker JE, Viebahn CS, Jakubowski A, et al. Tumor necrosis factor-like weak inducer of apoptosis is a mitogen for liver progenitor cells. Hepatology 2010;52:291-302. [PubMed]
- Ochoa B, Syn WK, Delgado I, et al. Hedgehog signaling is critical for normal liver regeneration after partial hepatectomy in mice. Hepatology 2010;51:1712-23. [PubMed]
- Karaca G, Swiderska-Syn M, Xie G, et al. TWEAK/Fn14 signaling is required for liver regeneration after partial hepatectomy in mice. PLoS One 2014;9:e83987. [PubMed]
- Tirnitz-Parker JE, Viebahn CS, Jakubowski A, et al. Tumor necrosis factor-like weak inducer of apoptosis is a mitogen for liver progenitor cells. Hepatology 2010;52:291-302. [PubMed]
- Bird TG, Lu WY, Boulter L, et al. Bone marrow injection stimulates hepatic ductular reactions in the absence of injury via macrophage-mediated TWEAK signaling. Proc Natl Acad Sci U S A 2013;110:6542-7. [PubMed]
- Cai Y, Zheng H, Gong W, et al. The role of hedgehog signaling pathway in liver regeneration. Hepatogastroenterology 2011;58:2071-6. [PubMed]
- Hanaoka J, Shimada M, Utsunomiya T, et al. Significance of sonic hedgehog signaling after massive hepatectomy in a rat. Surg Today 2013;43:300-7. [PubMed]
- Swiderska-Syn M, Syn WK, Xie G, et al. Myofibroblastic cells function as progenitors to regenerate murine livers after partial hepatectomy. Gut 2014;63:1333-44. [PubMed]
- Yang L, Wang Y, Mao H, et al. Sonic hedgehog is an autocrine viability factor for myofibroblastic hepatic stellate cells. J Hepatol 2008;48:98-106. [PubMed]
- Omenetti A, Syn WK, Jung Y, et al. Repair-related activation of hedgehog signaling promotes cholangiocyte chemokine production. Hepatology 2009;50:518-27. [PubMed]
- Choi SS, Omenetti A, Witek RP, et al. Hedgehog pathway activation and epithelial-to-mesenchymal transitions during myofibroblastic transformation of rat hepatic cells in culture and cirrhosis. Am J Physiol Gastrointest Liver Physiol 2009;297:G1093-106. [PubMed]
- Corbit KC, Aanstad P, Singla V, et al. Vertebrate Smoothened functions at the primary cilium. Nature 2005;437:1018-21. [PubMed]
- Grzelak CA, Martelotto LG, Sigglekow ND, et al. The intrahepatic signalling niche of hedgehog is defined by primary cilia positive cells during chronic liver injury. J Hepatol 2014;60:143-51. [PubMed]
- Short J, Ove P. Synthesis of an hypothesis advocating a prominent role for the thyroid hormones in mammalian liver cell proliferation in vivo. Cytobios 1983;38:39-49. [PubMed]
- Leffert HL, Alexander NM. Thyroid hormone metabolism during liver regeneration in rats. Endocrinology 1976;98:1241-7. [PubMed]
- Bockhorn M, Frilling A, Benko T, et al. Tri-iodothyronine as a stimulator of liver regeneration after partial and subtotal hepatectomy. Eur Surg Res 2007;39:58-63. [PubMed]
- László V, Dezso K, Baghy K, et al. Triiodothyronine accelerates differentiation of rat liver progenitor cells into hepatocytes. Histochem Cell Biol 2008;130:1005-14. [PubMed]
- Paku S, Schnur J, Nagy P, et al. Origin and structural evolution of the early proliferating oval cells in rat liver. Am J Pathol 2001;158:1313-23. [PubMed]
- Yovchev MI, Zhang J, Neufeld DS, et al. Thymus cell antigen-1-expressing cells in the oval cell compartment. Hepatology 2009;50:601-11. [PubMed]
- Steiling H, Werner S. Fibroblast growth factors: key players in epithelial morphogenesis, repair and cytoprotection. Curr Opin Biotechnol 2003;14:533-7. [PubMed]
- Takase HM, Itoh T, Ino S, et al. FGF7 is a functional niche signal required for stimulation of adult liver progenitor cells that support liver regeneration. Genes Dev 2013;27:169-81. [PubMed]
- Kim W, Kim M, Jho EH. Wnt/β-catenin signalling: from plasma membrane to nucleus. Biochem J 2013;450:9-21. [PubMed]
- Miao CG, Yang YY, He X, et al. Wnt signaling in liver fibrosis: progress, challenges and potential directions. Biochimie 2013;95:2326-35. [PubMed]
- Hu M, Kurobe M, Jeong YJ, et al. Wnt/beta-catenin signaling in murine hepatic transit amplifying progenitor cells. Gastroenterology 2007;133:1579-91. [PubMed]
- Camargo FD, Gokhale S, Johnnidis JB, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol 2007;17:2054-60. [PubMed]
- Dong J, Feldmann G, Huang J, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 2007;130:1120-33. [PubMed]
- Van Hul NK, Abarca-Quinones J, Sempoux C, et al. Relation between liver progenitor cell expansion and extracellular matrix deposition in a CDE-induced murine model of chronic liver injury. Hepatology 2009;49:1625-35. [PubMed]
- Lorenzini S, Bird TG, Boulter L, et al. Characterisation of a stereotypical cellular and extracellular adult liver progenitor cell niche in rodents and diseased human liver. Gut 2010;59:645-54. [PubMed]
- Lozoya OA, Wauthier E, Turner RA, et al. Regulation of hepatic stem/progenitor phenotype by microenvironment stiffness in hydrogel models of the human liver stem cell niche. Biomaterials 2011;32:7389-402. [PubMed]
- Kallis YN, Robson AJ, Fallowfield JA, et al. Remodelling of extracellular matrix is a requirement for the hepatic progenitor cell response. Gut 2011;60:525-33. [PubMed]
- Ruddell RG, Knight B, Tirnitz-Parker JE, et al. Lymphotoxin-beta receptor signaling regulates hepatic stellate cell function and wound healing in a murine model of chronic liver injury. Hepatology 2009;49:227-39. [PubMed]
- Subrata LS, Voon DC, Yeoh GC, et al. TNF-inducible expression of lymphotoxin-β in hepatic cells: an essential role for NF-κB and Ets1 transcription factors. Cytokine 2012;60:498-504. [PubMed]
- Kuramitsu K, Sverdlov DY, Liu SB, et al. Failure of fibrotic liver regeneration in mice is linked to a severe fibrogenic response driven by hepatic progenitor cell activation. Am J Pathol 2013;183:182-94. [PubMed]
- Dwyer BJ, Olynyk JK, Ramm GA, et al. TWEAK and LTβ Signaling during Chronic Liver Disease. Front Immunol 2014;5:39. [PubMed]
- Dechêne A, Sowa JP, Gieseler RK, et al. Acute liver failure is associated with elevated liver stiffness and hepatic stellate cell activation. Hepatology 2010;52:1008-16. [PubMed]
- Weissman IL, Anderson DJ, Gage F. Stem and progenitor cells: origins, phenotypes, lineage commitments, and transdifferentiations. Annu Rev Cell Dev Biol 2001;17:387-403. [PubMed]
- Morrison SJ, Kimble J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature 2006;441:1068-74. [PubMed]
- Best J, Dolle L, Manka P, et al. Role of liver progenitors in acute liver injury. Front Physiol;4:258.
- Russo FP, Parola M. Stem and progenitor cells in liver regeneration and repair. Cytotherapy 2011;13:135-44. [PubMed]
- Maitra A, Arking DE, Shivapurkar N, et al. Genomic alterations in cultured human embryonic stem cells. Nat Genet 2005;37:1099-103. [PubMed]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663-76. [PubMed]
- Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007;318:1917-20. [PubMed]
- Zhao T, Zhang ZN, Rong Z, et al. Immunogenicity of induced pluripotent stem cells. Nature 2011;474:212-5. [PubMed]
- Dollé L, Best J, Empsen C, et al. Successful isolation of liver progenitor cells by aldehyde dehydrogenase activity in naïve mice. Hepatology 2012;55:540-52. [PubMed]
- Hao PP, Lee MJ, Yu GR, et al. Isolation of EpCAM(+)/CD133 (-) hepatic progenitor cells. Mol Cells 2013;36:424-31. [PubMed]
- Yovchev MI, Dabeva MD, Oertel M. Isolation, characterization, and transplantation of adult liver progenitor cells. Methods Mol Biol 2013;976:37-51. [PubMed]
- Yasui O, Miura N, Terada K, et al. Isolation of oval cells from Long-Evans Cinnamon rats and their transformation into hepatocytes in vivo in the rat liver. Hepatology 1997;25:329-34. [PubMed]
- Cardinale V, Wang Y, Carpino G, et al. Multipotent stem/progenitor cells in human biliary tree give rise to hepatocytes, cholangiocytes, and pancreatic islets. Hepatology 2011;54:2159-72. [PubMed]
- Oikawa T, Kamiya A, Zeniya M, et al. Sal-like protein 4 (SALL4), a stem cell biomarker in liver cancers. Hepatology 2013;57:1469-83. [PubMed]
- Stachelscheid H, Urbaniak T, Ring A, et al. Isolation and characterization of adult human liver progenitors from ischemic liver tissue derived from therapeutic hepatectomies. Tissue Eng Part A 2009;15:1633-43. [PubMed]
- Gerlach JC, Over P, Turner ME, et al. Perivascular mesenchymal progenitors in human fetal and adult liver. Stem Cells Dev 2012;21:3258-69. [PubMed]
- Crisan M, Corselli M, Chen CW, et al. Multilineage stem cells in the adult: a perivascular legacy? Organogenesis 2011;7:101-4. [PubMed]
- Cordeiro-Spinetti E, de Mello W, Trindade LS, et al. Human bone marrow mesenchymal progenitors: perspectives on an optimized in vitro manipulation. Front Cell Dev Biol 2014;2:7. [PubMed]
- Baer PC. Adipose-derived mesenchymal stromal/stem cells: An update on their phenotype in vivo and in vitro. World J Stem Cells 2014;6:256-65. [PubMed]
- Iftimia-Mander A, Hourd P, Dainty R, et al. Mesenchymal stem cell isolation from human umbilical cord tissue: understanding and minimizing variability in cell yield for process optimization. Biopreserv Biobank 2013;11:291-8. [PubMed]
- De Coppi P, Bartsch G Jr, Siddiqui MM, et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol 2007;25:100-6. [PubMed]
- Secunda R, Vennila R, Mohanashankar AM, et al. Isolation, expansion and characterisation of mesenchymal stem cells from human bone marrow, adipose tissue, umbilical cord blood and matrix: a comparative study. Cytotechnology 2014. [Epub ahead of print]. [PubMed]
- Crapnell K, Blaesius R, Hastings A, et al. Growth, differentiation capacity, and function of mesenchymal stem cells expanded in serum-free medium developed via combinatorial screening. Exp Cell Res 2013;319:1409-18. [PubMed]
- Ochiya T, Yamamoto Y, Banas A. Commitment of stem cells into functional hepatocytes. Differentiation 2010;79:65-73. [PubMed]
- Lanzoni G, Oikawa T, Wang Y, et al. Concise review: clinical programs of stem cell therapies for liver and pancreas. Stem Cells 2013;31:2047-60. [PubMed]
- Volarevic V, Nurkovic J, Arsenijevic N, et al. Concise review: Therapeutic potential of mesenchymal stem cells for the treatment of acute liver failure and cirrhosis. Stem Cells 2014;32:2818-23. [PubMed]
- Bae SH. Clinical application of stem cells in liver diseases. Korean J Hepatol 2008;14:309-17. [PubMed]


