Contribution of bone marrow-derived fibrocytes to liver fibrosis
Introduction
Liver fibrosis and cirrhosis result from the pathological response to chronic liver injury. Liver fibrosis is characterized by intensive remodeling of liver tissue, formation of fibrous scar and appearance of collagen type I producing myofibroblasts (1). Hepatic myofibroblasts, which exist only in the damaged liver, exhibit stellate or spindle morphology, and are characterized by expression of α-smooth muscle actin (α-SMA), non-muscle myosin, fibronectin, vimentin, and collagen type I [the major component of extracellular matrix proteins (ECM) emerging in fibrotic liver]. Although heterogeneous in their origin, hepatic myofibroblasts share similar cellular characteristics, such as expression of α-SMA, collagen-α1(I), and other cytoskeletal proteins. To our current knowledge, there are three sources of hepatic myofibroblasts which critically contribute to liver fibrosis of distinct etiologies: hepatic stellate cells (HSCs), portal fibroblasts (PFs) and bone marrow (BM)-derived collagen producing fibrocytes (2,3) (Figure 1). In response to various types of injury, the composition of myofibroblasts appears to be different.
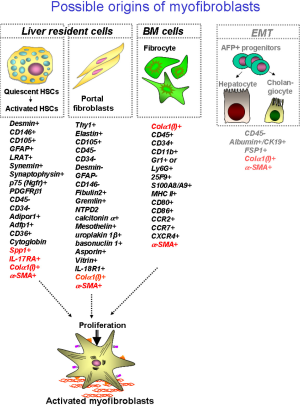
Recent studies have identified that HSCs are the major source of myofibroblasts which are induced during chronic toxic liver injury, such as hepatitis B virus (HBV) or hepatitis c virus (HCV) infection in patients, and alcoholic liver disease (ALD) (4). Similar results were obtained using experimental models of toxic liver injury in mice, such as administration of hepatotoxin carbon tertachloride (CCl4) or intragastric alcohol feeding (Tsukamoto-French model), in which injured hepatocytes undergo massive apoptosis or necrosis (5,6). Apoptotic hepatocytes release factors that stimulate recruitment of inflammatory cells to the site of injury and activation of BM-derived and liver resident (Kupffer cells) macrophages, which in turn, secrete pro-fibrogenic and pro-inflammatory cytokines like transforming growth factor β 1 (TGF-β1), interleukin 6 (IL-6), interleukin 1 β (IL-1β) and tumor necrosis factor α (TNF-α) causing rapid activation of quiescent hepatic stellate cells (qHSCs) to activated hepatic stellate cells (aHSCs)/myofibroblasts (1). Under physiological conditions qHSCs reside in the space of Disse (designated between hepatocytes and endothelial cells), store retinoids in lipid droplets, and express neural markers, such as glial fibrillar acidic protein (GFAP), synaptophisin, and nerve growth factor receptor p75 (1,7,8). In response to injury, qHSCs downregulate Vitamin A-containing lipid droplets and neural markers, and differentiate into collagen-α1(I) and α-SMA-expressing myofibroblasts (1,9), which migrate to the portal and peri-portal areas and deposit extracellular matrix proteins (ECM) to form a fibrous scar to toxic liver injury. Similar activation of aHSCs/myofibroblasts has been implicated in the pathogenesis of alcohol-induced liver injury (10). However, alcohol-induced toxic liver injury has certain distinctive pathogenic characteristics: since mice are fed with high fat + high cholesterol diet in the presence or absence of alcohol, development of hepatic steatosis and steatohepatitis are the prerequisite of ethanol-mediated liver fibrosis (10-14). Hence, aHSCs have been demonstrated to be the primary source of collagen type I-producing myofibroblasts (4,15,16). HSC-derived myofibroblasts can be identified by residual expression of GFAP and vitamin A, and expression of desmin, PDGFRIβ and p75 (which are absent in myofibroblasts of other origins) (10,17,18).
In turn, portal fibroblasts are believed to be a primary population of liver-resident mesenchymal cells that responds to cholestatic injury by giving rise to hepatic myofibroblasts, such as is observed during primary and secondary biliary cirrhosis in patients, or obstruction of biliary tract following ligation of the common bile duct ligation (BDL) in experimental mouse models of cholestatic fibrosis (19-22). Pathogenesis of biliary fibrosis (although not fully understood), is characterized by dysregulation of cholangiocyte proliferation, “ductular reaction” and rapid formation of periportal fibrosis (23-25). It is believed that decreased bile flow causes critical damage to the biliary epithelial cells, inducing secondary damage to hepatocytes [that release alkaline phosphotase (AP) and factors], which facilitate activation of liver resident myofibroblasts. Recent studies have suggested that activated portal fibroblasts (aPFs) are the first responders to cholestatic injury, and significantly contribute to collagen type I deposition at the onset of liver injury. Portal fibroblasts normally comprise a small population of the fibroblastic cells that surround the portal vein to maintain integrity of the portal tract. They were first described as “mesenchymal cells not related to sinusoids”, and since then have been called “periductular fibroblasts” or portal/periportal mesenchymal cells” (19) and are implicated by association in the pathogenesis of cholestatic liver injury. In response to chronic injury, portal fibroblasts may proliferate, differentiate into α-SMA-expressing myofibroblasts, and synthesize extracellular matrix (19-22). However, aPFs are not the only source of hepatic myofibroblasts in BDL-injured livers. Recent studies suggested that early activated PFs release factors (such as IL-13) that stimulate activation of HSCs in the mouse model of cholestatic liver injury, indicating that BDL-activated HSCs exhibit more similarity to aPFs than to CCl4-activated HSCs (17). Only a few markers of PFs are available to identify them in the myofibroblast population, including gremlin, Thy-1, fibulin-2, IL-6, elastin, the ecto-AT-Pase nucleoside triphosphate diphosphohydrolase-2 (NTPD2), and cofilin-1. Recently several novel markers of aPFs have been identified, such as mesothelin, asporin, and uroplakin 1β (17), however the importance of these proteins for aPF functions remains to be characterized. In addition, the lack of desmin, cytoglobin, α2-macroglobulin, neural proteins (GFAP, p75, synaptophysin), and lipid droplets distinguishes PFs from HSCs (1,26-30).
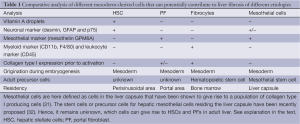
Mesothelial cells are defined as cells in the liver capsule that have been shown to give rise to a population of collagen type I producing cells (31) (Table 1). However, it remains unclear if these mesothelial cells can significantly contribute to liver fibrosis. Based on the finding by Li et al. (31), these mesothelial cells give rise to myofibroblasts that are in a close proximity to the liver capsule, but do not migrate deep into liver parenchyma.
Full table
Fibrocytes were first described by Bucala et al., and are defined by the simultaneous expression of CD45 and collagen type I (33-35). Fibrocytes possess dual characteristics of fibroblasts (expression of collagen type I, fibronectin and vimentin) and hematopoietic cells (CD45, CD34, MHCII, CD11b, Gr1, Ly6c, CD54, CD80, CD86, CCR2, CCR1, CCR7, CCR5) (36,37). Under physiological conditions, fibrocytes express CD45, CD34, and in culture exhibit a spindle-like shape. In response to injury (including liver injury), or stimulation by TGF-β, fibrocytes downregulate expression of hematopoietic markers and rapidly differentiate into α-SMA+ myofibroblasts which express collagen type I and obtain a stellate shape (37,38). Due to the ability to give rise to fibrogenic myofibroblasts, fibrocytes were implicated in the pathogenesis of skin, lung, kidney, and liver fibrosis (35,39-47). To this date, the differentiation into myfobroblasts is believed to be the main function of activated fibrocytes. In addition to collagen type I deposition, other functions of fibrocytes have been described. Fibrocytes were implicated in antigen presentation to naive T cells prompting their proliferation. Consistently, fibrocytes express of major histocompatibility complex class II (MHC II) and co-stimulatory molecules, CD80 and CD86. It has also been suggested that under certain circumstances (such as sepsis or bacterial infection) fibrocytes may mediate anti-microbia functions, and prevent bacterial spread by entrapping and killing bacteria. Interestingly, BM fibrocytes were shown to lack the ability to phagocytose bacteria (unlike macrophages and neutrophils) (48), but similar to macrophages and neutrophils can kill bacterial by releasing intracellular DNA nets which contain anti-microbial enzymes (49,50). Furthermore, stimulation of fibrocytes with macrophage-differentiating factors (M-CSF and GM-CSF) results in their differentiation into macrophages and dendritic cells (DCs) (48). Since fibrocytes can be rapidly differentiated into myofibroblasts in response to TGF-β1, recent studies have suggested that fibrocytes possess certain plasticity characteristics for precursor cell phenotype (48,51,52).
Another population of BM cells that can potentially give rise to hepatic myofibroblasts is mesenchymal progenitors (53,54). Hence, it remains unclear if mesenchymal cells (also known as BM-derived mesenchymal stem cells, circulating mesenchymal cells) possess pro-fibrotic or anti-fibrotic properties. It has been suggested (55-58) that mesenchymal cells migrate to damaged liver where they differentiate into fibrogenic myofibroblasts. However, several studies suggested that adoptive transfer of mesenchymal stem cells facilitates regression of liver fibrosis (59-62). Cell fate mapping of BM-derived mesenchymal stem cells will be needed to further examine the role of these cells in liver fibrosis (63).
This review will discuss the current understanding of fibrocyte biology and outline future prospects of using fibrocytes as targets for anti-fibrotic therapy.
Recruitment of fibrocytes to fibrotic tissues
Fibrocytes, designated as CD45 and collagen type I expressing hematopoietic cells, comprise a small population in the BM (33,34,36,64,65). Under physiological conditions, only a few fibrocytes can be detected in the peripheral blood or tissues. It is believed that fibrocytes contribute to wound healing and maintenance of tissue integrity, and therefore, play a critical role in matrix remodeling and cellular homeostasis (51). However, chronic injury results in disregulation of physiological process, causing rapid proliferation and egress of fibrocytes from the BM and homing to the site of injury. In response to injury, circulating fibrocytes populate the damaged tissue, where they contribute to ECM deposition (66). The number of fibrocytes recruited to a fibrosing organ seems to vary dependent on the tissue and type of injury (2,67). In patients, recruitment of fibrocytes to the scarring foci have been well documented, in part due to the availability of high quality anti-human CD45 and pro-collagen type I antibodies readily available for immunohistochemistry and flow cytometry. Thus, development of nephrogenic fibrosing dermopathy (NFD), a severe skin fibrosis caused by gadolinium intoxication in patients undergoing repetitive MRI contrast administration, has been shown to be mediated by fibrocytes (33,68,69), which are often stained positive for iron (70). Furthermore, high numbers of fibrocytes were detected in lungs of patients with pulmonary fibrosis, and correlated with the severity of lung fibrosis. In addition, increased levels of circulating fibrocytes were often observed in peripheral blood of these patients, suggesting that circulating fibrocytes may serve as a biomarker of pulmonary fibrosis progression (71-75). Fibrocytes are also detected in human fibrosing disorders such as bronchial asthma, and burns (76,77), and their presence is also detected by immunohistochemistry in kidney biopsy specimens from patients with chronic kidney disease (78,79). The number of infiltrated fibrocytes in the interstitium correlated well with the severity of tubulointerstitial lesions, such as interstitial fibrosis. In particular, there was an inverse correlation between the number of interstitial fibrocytes and kidney function at the time of biopsy (78). Finally, circulating fibrocytes were implicated to serve as a marker of liver fibrosis in chronic hepatitis C (80). Fibrocytes were also shown to contribute to the pathogenesis of Crohn’s disease (81).
Hence, the data obtained in patients relies majorly on the specificity of the immunoreactivity of anti-human antibodies and their conjugates. Therefore, exploration of experimental models of fibrogenesis of different organs and systems is critical to characterize the complex role of fibrocytes in fibrosis, the pathways of their activations, and mechanism of their action. At the present time, the contribution of fibrocytes to fibrosis of different organs remains unresolved. Based on mouse models of fibrosis of parenchymal organs, fibrocytes comprise 5% to 20% of the population of fibrogenic myofibroblast in fibrotic organs (2). The highest number of recruited fibrocytes has been observed in bleomycin-injured lungs, and corresponded to 25% to 50% of fibrogenic myofibroblasts (2,40,47,72,73,82,83). These data have been demonstrated by at least two independent scientific groups, suggesting that fibrocyte recruitment may significantly contribute to lung fibrosis. Next, fibrocytes were reported to be recruited to fibrotic liver in response to two models of hepatic fibrosis, one which mimics obstruction of the common biliary tract (BDL), and the second toxic liver injury, such as exposure to carbon tetrachloride (CCl4) (48,84,85). The results obtained by our group indicated that fibrocytes [designated as BM-derived cells expressing reporter collagen-α1(I)-green fluorescent protein (GFP) in real time] contribute to approximately 5% to 6% of collagen expressing cells in BDL- or CCl4-injurd liver (84). More recent studies have suggested that fibrocytes may not serve as a significant source of collagen type I in fibrotic liver, and cannot be considered as major contributers to ECM deposition in response to BDL or CCl4 injury because: (I) low number of CD45+ Col+ fibrocytes detected in fibrotic liver [Off note, if expression of fibrocyte markers, such as CD45 and/or collagen type I, changes in the course of injury, the number of cells originated from fibrocytes (fibrocyte progeny) may be much more significantly present in fibrotic liver, but remain undetected by available methods]; (II) isolated fibrocytes were shown to express on average ten times less collagen-α1(I) than hepatic myofibroblasts originated from aHSCs (48). Taken together, recruitment of fibrocytes is a prerequisite of fibrogenic liver injury, however, contribution of fibrocytes to hepatic myofibroblasts remains questionable. Although fibrocytes may not serve as a major source of collagen type I deposition in fibrotic liver caused by BDL or CCl4 (3), this conclusion may not extend to some genetic defects causing liver fibrosis in mice. Sclerosing cholangitis caused by genetic mutation of multidrug resistance genes (Mdr2-/-, Abcb4-/-mice), results in cholestatic liver injury and subsequent recruitment and activation of BM-derived fibrocytes, portal fibroblasts and HSCs causing biliary fibrogenesis in Abcb4-/-mice. Abcb4 deficiency results in a significant flux of fibrocyts to the liver (up to 25% of total myofibroblasts) (31). Although, the nature of these differences remains unresolved, this phenomenon can be, in part, explained by differential expression of multiple genes in Abcb4-/-mice (versus wild type mice), causing a significant amplification of immunoregulatory function of fibrocytes in these Abcb4-/-mice (33,34). Notably, the specific model (i.e., the etiology of tissue injury and timeframe) and the method of analysis (i.e., applied surrogate parameters reflecting fibrosis or fibrogenesis, respectively) are important factors for understanding of the role of BM-derived cells in the pathogenesis of fibrosis (31). In addition, the methods of fibrocyte detection and monitoring can be critical in dissecting these discrepancies in experimental findings, and will be discussed below. Similarly, the contribution of fibrocytes to kidney fibrosis remains controversial. According to Sakai et al. (43,44,78,79), BM-derived fibrocytes populate fibrotic kidney giving rise to approximately 15% of renal myofibroblasts. In turn, the data obtained by Lin et al. (39) suggests that fibrocytes only minimally contribute to renal fibrosis, unlike renal pericytes (the cells with functions similar to that detected in hepatic Stellate cells, often referred to as hepatic pericytes).
Migration of fibrocytes is restricted to fibrotic organ (with one exception: recruitment of fibrocytes to the spleen has been documented in experimental models of liver and kidney fibrosis) (43,48). In concordance, adoptive transfer of fibrocytes also results in their specific homing to the damaged organ (48,86,87). It remains unclear how fibrocytes migrate specifically to the damaged organ. Several mechanisms have been suggested to regulate fibrocyte recruitment. Development of fibrosis, including liver fibrosis, is associated with elevated levels of biologically active TGF-β1, and release of intestinal lipopolysaccharide (LPS) into circulation (88,89). These factors may serve as primary fibrocyte chemoattractants and play a critical role in fibrocyte recruitment. In vitro transmigration assay has demonstrated that fibrocyte migration can be mediated by TGF-β1, and LPS (48). Thus, infection of mice with TGF-β1 expressing adenoviral vector (that targets hepatocytes) resulted in rapid recruitment of fibrocytes to the liver (and spleen) (47,90,91). TGF-β1 was also shown to trigger in vivo fibrocyte mobilization into fibrotic liver, lung and kidneys, suggesting that regulation of fibrocyte migration by TGF-β1 might be a general characteristic of fibrogenic injury of the liver and other parenchymal organs (44,82,92,93). Furthermore, similar to other hematopoietic cells, fibrocytes express chemokine receptors CCR1, CCR2 (85), CCR3 CCR5, CCR7 and CXCR3 but not CCR4, CCR6 or CXCR3 (36), which mediate their homing to fibrotic foci. Thus, fibrocytes devoid of CCR1 or CCR2 expression exhibit a defect in homing to fibrotic liver (85). Meanwhile, CCR2 and CCR7 (but not CCR1) were shown to be important for fibrocyte recruitment to fibrotic kidneys and lungs (43,45,47,90,91,94). The importance of CXCL12 (stromal derived factor 1, SDF-1) in fibrocyte recruitment to fibrosing lungs and skin has been previously demonstrated, suggesting that recruitment of fibrocytes is regulated on multiple levels. Both hypoxia-induced and growth factor-induced expression of CXCR4, a receptor for CXCL12 regulates fibrocyte homing to fibrotic liver and can be blocked by addition specific inhibitors of PI3-kinase and mTOR to fibrocyte cultures. Consistently, bleomycin-induced pulmonary fibrosis was attenuated when mice were treated with the mTOR inhibitor rapamycin, and correlated with the reduced numbers of CXCR4-expressing fibrocytes in the peripheral blood and lung as well as reduced lung collagen deposition (86). Recent studies have demonstrated that Tregs, regulatory T cells, play a critical role in downregulation of CXCL12 production and inhibition of fibrocyte recruitment along the CXCL12-CXCR4 axis in injured lungs, suggesting that Treg may reduce fibroproliferation (95).
Approaches to study fibrocytes
Several approaches have been used to isolate and culture fibrocytes, and also detect and monitor migration of BM-derived fibrocytes into a fibrotic organ. Fibrocytes can be enriched in vitro by isolation of circulating monocytes from peripheral blood (33,34). It is believed that fibrocytes differentiate from a subset of CD11b+, CD115+ and Gr1+ monocytes, and this process is regulated by T cell-derived cytokines (96). In culture, all fibrocytes isolated from circulating blood exhibit spindle shape and upregulate the myofibroblast marker α-SMA. During 2 to 7 days in culture (36), fibrocyte-derived myofibroblasts may retain myeloid marker CD11b and CD14, but gradually downregulate these markers following prolonged culturing (66,72,73,97). It is believed that fibrocytes differentiate from a subset of CD11b+, CD115+ and Gr1+ monocytes, and this process is regulated by T cell-derived cytokines (96). Thus, IL-4 and IL-13 from Th2 cells promote outgrowth of fibrocytes from CD14+ precursors, while interferon-γ (IFN-γ) and IL-12 produced by Th1 inhibit fibrocyte outgrowth (98). In vitro studies of fibrocytes, yet present a unique possibility to study fibrocyte biology and signaling (99), also have serious limitations. Thus, meticulous comparison of markers of cultured fibrocytes, monocytes and macrophages using immunocytochemistry revealed that macrophages exhibit immunoreactivity with anti-pro-collagen type I antibody (100), and can be distinguished from CD45RO+, 25F9+, and S100A8/A9+ fibrocytes by expression of PM-2K. Hence, the gene expression profiling of freshly isolated activated macrophages and fibrocytes suggested that fibrocytes express on average 2.5 fold more collagen type I mRNA, indicating that elevated expression of Col1a1 and Col1a2 are distinctive features of fibrocytes (48). In mice, fibrocytes can be detected from a pool of BM-derived cells by co-staining with CD45 and pro-collagen type I using flow cytometry (40,82,101). To distinguish hematopoietic cells from tissue-resident cells, many studies have utilized bone marrow transplantation (BMT) using reporter mice (ubiquitously expressing fluorescent protein in all hematopoietic cells) as donors (67). In addition, the fate mapping of the whole hematopoietic cellular lineages have been employed to track their migration to damaged skin using Vav-1-Cre mice crossed with the reporter mice. Fibrocytes were identified in these mice as collagen I expressing cells expressing certain markers of myeloid lineage, including low density expression of CD11b and CD45 (102). Our group has developed another functional method to distinguish fibrocytes from liver resident fibrogenic myofibroblasts and other BM-derived cells (84,103-105). This method utilizes transgenic reporter collagen-α1(I)-GFP mice in which every cell producing collagen type I upregulates expression of GFP. Therefore, specific labeling of BM-derived CD45+ collagen-α1(I)+ fibrocytes in real time can be achieved in BM chimeric mice generated by transplantation of the collagen-α1(I)-GFP+ (Col-GFP) BM into lethally irradiated wild type recipient mice, since expression of Col-GFP can be observed in fibrocytes but not in other hematopoietic cells [such as activated macrophages (100)]. These BM chimeric Col-GFP→wt mice were considered to be a useful tool to monitor fibrocyte transmigration from the BM to peripheral tissues under physiological conditions, and in response to fibrogenic injury. Toxic and cholestatic liver injury caused rapid recruitment of BM-derived fibrocytes (56,57) to fibrotic livers of Col-GFP→wt mice. Using Col-GFP→wt mice, we have identified that fibrogenic liver injury activates several populations of fibrocytes: hepatic (84), splenic (104) and BM CD45+Col+ fibrocytes (67,106). Col-GFP→wt mice were successfully used to monitor fibrocyte flux into fibrotic liver, but could also be used to compare the contribution of fibrocytes to the fibrogenesis of other organs and tissues. Thus, other studies have used this approach and methodology to visualize fibrocytes recruited to fibrosing kidney (39). To assess the in vivo fibrocyte function and differentiation, a gender mismatched BMTof BM-derived collagen type I producing cells are utilized in mouse models of fibrosis (84). All of these studies support the the growing evidence of involvement of BM-derived fibrocytes in wound healing, scarring and fibrosis, suggesting that fibrocytes play an important role in fibrogenesis.
Fibrocytes detected at extrahepatic sites in response to liver fibrosis
Studies using BMT in mice have established that the BM is the primary source of fibrogenic fibrocytes. Under physiological conditions fibrocytes are primarily located in the BM, where they comprise a small subset (0.1%) of mononuclear cells, which proliferate and transmigrate with the blood stream in response to injury (37). Fibrocytes have been isolated from fibrotic tissues, spleens and peripheral blood (34,37). Development of liver fibrosis is strongly associated with elevated levels of TGF-β1, increased intestinal permeability and release of endogenous LPS. In addition to the injured organ, recruitment of CD45+Col+ fibrocytes to the spleen has been documented in liver (84,107) and kidney fibrosis (43). Hepatotoxic injury (CCl4), TGF-β1, and endogenous LPS trigger migration of fibrocytes from the BM to the spleen and liver (104). Moreover, the spleen functions as a major reservoir of immature fibrocytes (108). Splenic CD45+Col+ fibrocytes express myeloid markers and resemble CD115+CD11b+ monocytes (104). Splenic fibrocytes express myeloid cell markers CD45, CD11b and Ly6c and expression of collagen I, similar to that observed in BM fibrocytes, but in spleen they do not transdifferentiate into myofibroblasts in vivo, consistently, liver fibrosis does not cause ECM deposition in the spleen. Although the biological significance of splenic fibrocytes is not understood, our recent study suggests that CD45+Col+ fibrocytes are capable of differentiating according to their microenvironment, giving rise to different subtypes of fibrocyte-like cells with distinct roles during tissue repair and fibrosis (109). Consistent with this observation, infection with Listeria monocytogenes (Lm) also causes migration of fibrocytes specifically to Lm-infected spleen and liver, indicating their potential role in innate immunity. Splenic fibrocytes can uniquely upregulate a variety of antimicrobial factors (myeloperoxidase, cathelicidin antimicrobial peptide (mCRAMP), defensins) (104) to entrap and kill bacteria (Lm) (49,104,110-112). Although the antimicrobial properties of fibrocytes, are aimed at stopping infection (113), release of nuclear DNA and lysosomal peptides into the extracellular space facilitates inflammation (114). In addition, upon migration to the spleen, fibrocytes strongly upregulate expression of MHC II (106) and mediate adaptive immunity by presenting antigens to naive T cells (64,104,115), causing their rapid proliferation. The diverse functions of splenic and hepatic fibrocytes may imply that circulating fibrocytes are not terminally differentiated. In other words, they may comprise a mixed population of myeloid progenitors at different stages of maturation, which retain a potential to further differentiate into myofibroblasts, or antigen presenting cells within wounded tissue, or engraft into spleen to support innate and adaptive immune responses.
Differentiation of fibrocytes
Differentiation of fibrocytes from monocytes is regulated by FCγ receptors FCγRI (CD64) and FCγRII (CD32). FCγ receptors are broadly expressed on the immune cell membranes, and the recognition of IgG by FCγ receptors plays an important role in the antigen presenting process. The antigen-IgG complex in circulating blood is anchored by FCγ receptors, which initiate the internalization and trafficking of the antigen-IgG complex into the vesicle machineries. The antigens in the vesicles are separated from IgGs, and the epitopes are bound to MHC-I and MHC-II, which work together with CD80 and CD86 stimulating proliferation of cognate CD4 and CD8 T cells (116). To our current knowledge, there are four FCγ receptor isotypes, FCγI, FCγII, FCγIII (CD16) and neonatal FCγ receptor (FcRn). While the FCγ I-III are membrane receptors and mediate internalization of IgG, FcRn is an intracellular receptor which distinguishes between IgG and IgG-antigen complex and mediates epitope conservation within the endolysosomal machinery. All the monocytes express FCγ II and III, and only a small subset of monocytes express FCγ I, II and III (100,117,118). The mature macrophages express FCγII and FCγIII, but not FCγI in culture, while the predominant FCγ isotype on fibrocytes is FCγII receptor (100). The FCγ receptors inhibitor human Serum Amyloid P (hSAP) have been shown anti-fibrosis potential on kidney (119), skin and liver fibrosis by blocking fibrocyte differentiation from monocytes or have had anti-inflammatory effect on monocytes and macrophages.
It has been reported that fibrocyte growth and maturation is inhibited by hSAP (120). hSAP is an evolutionary highly conserved protein that is induced in the acute-phase response (121). hSAP is a member of the pentraxin family of proteins that includes C-reactive protein (CRP) (122-124). hSAP is produced by the liver as a 27-kDa protein, and secreted into the blood where it circulates as a stable 135-kDa pentamer (121,125,126). Interaction between hSAP and FcγRI and II regulates activation of srk-related tyrosine kinases, a key component in inhibition of fibrocyte differentiation into myofibroblasts (127). In addition, hSAP was shown to bind to apoptotic cells, DNA, and certain microorganisms (119). Due to the unique binding specificity and localization to the sites of injury, hSAP is implicated in blocking fibrosis of injured organs (119). hSAP was shown to successfully inhibit experimental fibrosis in lungs (128,129), kidneys (39,119), skin (130,131), and attenuate chronic lung infection caused by Pseudomonas aeruginosa (132,133). Based on these observations, we hypothesize that hSAP will inhibit fibrocyte functions in experimental liver fibrosis and provide new insight into the contribution of fibrocytes to liver fibrosis.
The dual role of fibrocytes during fibrogenesis
Activation and senescence/inactivation of myofibroblasts are regulated by factors secreted by recruited inflammatory cells and macrophage/monocytes to the site of injury (134). Fibrosis progression depends on the level of inflammation and production of TGF-β1 by myeloid cells. Fibrocytes contribute to tissue fibrosis not only by direct collagen deposition, but also by secreting pro-fibrogenic and pro-inflammatory cytokines. Accumulation of fibrocytes was observed within inflammatory lesions rather than in the fibrotic scar area (135). The cytokine profile of tissue fibrocytes includes TNF-α, IL-1β, IL10, TGF-b1 and M-CSF, and the chemokine profile is MIP-1α, MIP-1bβ, MIP-2, MCP-1 and vascular endothelial growth factor (97,102,135). Circulating fibrocytes enriched by CD34+ marker and stimulated with TNF-α and IL-1β respond by secretion of chemokines MIP-1α, MIP1β and MCP-1, IL-8 and GROa (97). Expression of the pro-inflammatory cytokines, TGF-β1 and TNF-α in tissue fibrocytes often correlates with the level of collagen expression (102,135). The majority of cytokines and chemokines that have been reported to be expressed in fibrocytes, promote tissue inflammation and facilitate leukocyte recruitment into the injured tissues. Interestingly, fibrocytes themselves have a potential to differentiate into mature macrophages upon M-CSF stimulation (48), suggesting that fibrocytes microenvironment might drive their differentiation, or significantly affect their function.
Fibrocytes possess plasticity characteristic for hematopoietic precursor cells
Expression of the precursor cell marker CD34 on fibrocytes supports the hypothesis that fibrocytes may possess a certain plasticity and retain characteristics of precursor cells (100). In concordance, upon migration into an injured organ, circulating fibrocytes gradually downregulate hematopoietic markers, including CD45 and CD34, and obtain myofibroblast-like markers, such as α-SMA and vimentin. Differentiation of fibrocytes into myofibroblasts has been documented in liver, lung, kidney and skin fibrosis. Hence, there is an emerging body of evidence that fibrocytes can also give rise to cells of myeloid lineages. Thus, in vitro stimulation of splenic (and BM) fibrocytes with M-CSF results in fibrocyte differentiation into fully functional macrophages, which upregulate markers of mature macrophages and are capable of phagocytosis. Similar to that, culturing of fibrocytes in the presence of GM-CSF drives fibrocyte differentiation towards dendritic cells (DCs). Interestingly, expression of collagen was downregulated in fibrocytes upon differentiation. In support of these in vitro results, adoptive transfer of CD45.2+ fibrocytes into sublethally irradiated CD45.1+ mice resulted in fibrocytes homing to the spleen where they engrafted and proliferated. Two weeks after transfer, fibrocytes and their progeny, identified by CD45.2 expression, constituted up to 5% of total splenocytes. They downregulate collagen-α1(I)-GFP reporter expression (indicative of downregulation of collagen type I expression in real time), and give rise to CD11b+, GR1+ and CD11c+ cells. Downregulation of the original markers makes it difficult to lineage trace the progeny of fibrocytes in tissues. The most recent study took advantage of Vav-1-Cre mice to distinguish all hematopoietic cells from those of non-hematopoietic origin. Use of single-cell transcriptional analysis in this mouse model revealed two discrete types of collagen I (Col I) expressing cells of hematopoietic lineage recruited into excisional skin wounds, CD45+CD11b+Col+ and CD45−CD11b−Col+ cells, suggesting that BM-derived fibrocytes can give rise to multipple populations in the injured tissue.
Fibrocytes differ from BM-derived mesenchymal stem cells. Both fibrocytes and mesenchymal stem cells (MSCs) are derived from the BM, but they differ significantly in expression of their cellular markers and functions. While fibrocytes contribute to the ECM deposition and secrete pro-inflammatory cytokines like IL-1β, IL-6, TNF-α and TGF-β1, MSCs are believed to attenuate scar formation and suppress inflammatory responses at the site of fibrotic lesion. MSCs were originaly observed in the stroma of BM (136), MSCs that are usually isolated by outgrowth of BM cells possess stem cell/progenitor properties and are characterized by their ability to differentiate into at least three cell populations: adipocytes, osteocytes and chondrocytes, in response to appropriate stimuli. Recently, the ability of MSCs to differentiate into skeletal muscle cells and neurons has been reported (137-139). There are several cell membrane markers that can be used to discriminate between fibrocytes and MSCs. MSCs lack hematopoietic cell markers CD14, CD34 and CD45, but express CD44, CD105 and CD90 (which were not found on fibrocytes). Unlike fibrocytes (that are capable of antigen presentation), MSCs inhibit proliferation of naive and memory T cells in a non-cognate manner, and such an inhibitory effect is proportional to the ratio of MSCs to T cells (140).
Conclusions
Recent studies have provided convincing evidence that fibrocytes play an important role in the fibrogenesis of parenchymal organs. Both TGF-β1 and LPS play a critical role in liver fibrogenesis, and these factors also appear to trigger fibrocyte recruitment to the injured liver, and promote their differentiation into collagen type I producing myofibroblasts. Fibrocytes were implicated in the pathogenesis of liver, lung, skin and kidney fibrosis. Meanwhile, fibrocytes recruited to the spleen in response to acute liver injury or infection are involved in regulation and mediation of innate immune responses rather than promoting in situ ECM deposition. Future studies will provide a better understanding of fibrocyte functions dependent on the microenvironment and type of injury. Fibrocytes may become a novel target for anti-fibrotic therapy.
Acknowledgements
We thank Karin Diggle for her critical reading of this manuscript.
Funding: This work was supported by the National Institutes of Health (DK088837, GM41804, AA15055, DK72237, AI0777802 P50 AA011999), the American Liver Foundation.
Disclosure: The authors declare no conflict of interest.
References
- Bataller R, Brenner DA. Liver fibrosis. J Clin Invest 2005;115:209-18. [PubMed]
- Kisseleva T, Brenner DA. Fibrogenesis of parenchymal organs. Proc Am Thorac Soc 2008;5:338-42. [PubMed]
- Kisseleva T, Brenner DA. Anti-fibrogenic strategies and the regression of fibrosis. Best Pract Res Clin Gastroenterol 2011;25:305-17. [PubMed]
- Iwaisako K, Brenner DA, Kisseleva T. What’s new in liver fibrosis? The origin of myofibroblasts in liver fibrosis. J Gastroenterol Hepatol 2012;27 Suppl 2:65-8. [PubMed]
- Kisseleva T, Brenner DA. Mechanisms of fibrogenesis. Exp Biol Med (Maywood) 2008;233:109-22. [PubMed]
- Brenner DA, Seki E, Taura K, et al. Non-alcoholic steatohepatitis-induced fibrosis: Toll-like receptors, reactive oxygen species and Jun N-terminal kinase. Hepatol Res 2011;41:683-6. [PubMed]
- Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev 2008;88:125-72. [PubMed]
- Friedman SL, Roll FJ, Boyles J, et al. Hepatic lipocytes: the principal collagen-producing cells of normal rat liver. Proc Natl Acad Sci U S A 1985;82:8681-5. [PubMed]
- Forbes SJ, Parola M. Liver fibrogenic cells. Best Pract Res Clin Gastroenterol 2011;25:207-17. [PubMed]
- Kisseleva T, Cong M, Paik Y, et al. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc Natl Acad Sci U S A 2012;109:9448-53. [PubMed]
- Tsukamoto H. Fat paradox in liver disease. Keio J Med 2005;54:190-2. [PubMed]
- Tsukamoto H, Mkrtchyan H, Dynnyk A. Intragastric ethanol infusion model in rodents. Methods Mol Biol 2008;447:33-48. [PubMed]
- Tsukamoto H, Zhu NL, Wang J, et al. Morphogens and hepatic stellate cell fate regulation in chronic liver disease. J Gastroenterol Hepatol 2012;27 Suppl 2:94-8. [PubMed]
- Ueno A, Lazaro R, Wang PY, et al. Mouse intragastric infusion (iG) model. Nat Protoc 2012;7:771-81. [PubMed]
- Abdelmegeed MA, Banerjee A, Yoo SH, et al. Critical role of cytochrome P450 2E1 (CYP2E1) in the development of high fat-induced non-alcoholic steatohepatitis. J Hepatol 2012;57:860-6. [PubMed]
- Kisseleva T, Brenner DA. Inactivation of myofibroblasts during regression of liver fibrosis. Cell Cycle 2013;12:381-2. [PubMed]
- Iwaisako K, Jiang C, Zhang M, et al. Origin of myofibroblasts in the fibrotic liver in mice. Proc Natl Acad Sci U S A 2014;111:E3297-305. [PubMed]
- Troeger JS, Mederacke I, Gwak GY, et al. Deactivation of hepatic stellate cells during liver fibrosis resolution in mice. Gastroenterology 2012;143:1073-83.e22.
- Dranoff JA, Wells RG. Portal fibroblasts: Underappreciated mediators of biliary fibrosis. Hepatology 2010;51:1438-44. [PubMed]
- Bhunchet E, Wake K. Role of mesenchymal cell populations in porcine serum-induced rat liver fibrosis. Hepatology 1992;16:1452-73. [PubMed]
- Desmoulière A, Darby I, Costa AM, et al. Extracellular matrix deposition, lysyl oxidase expression, and myofibroblastic differentiation during the initial stages of cholestatic fibrosis in the rat. Lab Invest 1997;76:765-78. [PubMed]
- Uchio K, Tuchweber B, Manabe N, et al. Cellular retinol-binding protein-1 expression and modulation during in vivo and in vitro myofibroblastic differentiation of rat hepatic stellate cells and portal fibroblasts. Lab Invest 2002;82:619-28. [PubMed]
- Jhandier MN, Kruglov EA, Lavoie EG, et al. Portal fibroblasts regulate the proliferation of bile duct epithelia via expression of NTPDase2. J Biol Chem 2005;280:22986-92. [PubMed]
- Wen JW, Olsen AL, Perepelyuk M, et al. Isolation of rat portal fibroblasts by in situ liver perfusion. J Vis Exp 2012;3669. [PubMed]
- Kruglov EA, Jain D, Dranoff JA. Isolation of primary rat liver fibroblasts. J Investig Med 2002;50:179-84. [PubMed]
- Clouzeau-Girard H, Guyot C, Combe C, et al. Effects of bile acids on biliary epithelial cell proliferation and portal fibroblast activation using rat liver slices. Lab Invest 2006;86:275-85. [PubMed]
- Knittel T, Kobold D, Saile B, et al. Rat liver myofibroblasts and hepatic stellate cells: different cell populations of the fibroblast lineage with fibrogenic potential. Gastroenterology 1999;117:1205-21. [PubMed]
- Goodpaster T, Legesse-Miller A, Hameed MR, et al. An immunohistochemical method for identifying fibroblasts in formalin-fixed, paraffin-embedded tissue. J Histochem Cytochem 2008;56:347-58. [PubMed]
- Dranoff JA, Kruglov EA, Robson SC, et al. The ecto-nucleoside triphosphate diphosphohydrolase NTPDase2/CD39L1 is expressed in a novel functional compartment within the liver. Hepatology 2002;36:1135-44. [PubMed]
- Bosselut N, Housset C, Marcelo P, et al. Distinct proteomic features of two fibrogenic liver cell populations: hepatic stellate cells and portal myofibroblasts. Proteomics 2010;10:1017-28. [PubMed]
- Li Y, Wang J, Asahina K. Mesothelial cells give rise to hepatic stellate cells and myofibroblasts via mesothelial-mesenchymal transition in liver injury. Proc Natl Acad Sci U S A 2013;110:2324-9. [PubMed]
- Rinkevich Y, Mori T, Sahoo D, et al. Identification and prospective isolation of a mesothelial precursor lineage giving rise to smooth muscle cells and fibroblasts for mammalian internal organs, and their vasculature. Nat Cell Biol 2012;14:1251-60. [PubMed]
- Bucala R. Circulating fibrocytes: cellular basis for NSF. J Am Coll Radiol 2008;5:36-9. [PubMed]
- Bucala R, Spiegel LA, Chesney J, et al. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med 1994;1:71-81. [PubMed]
- Grieb G, Steffens G, Pallua N, et al. Circulating fibrocytes--biology and mechanisms in wound healing and scar formation. Int Rev Cell Mol Biol 2011;291:1-19. [PubMed]
- Abe R, Donnelly SC, Peng T, et al. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol 2001;166:7556-62. [PubMed]
- Quan TE, Cowper S, Wu SP, et al. Circulating fibrocytes: collagen-secreting cells of the peripheral blood. Int J Biochem Cell Biol 2004;36:598-606. [PubMed]
- Quan TE, Bucala R. Culture and analysis of circulating fibrocytes. Methods Mol Med 2007;135:423-34. [PubMed]
- Lin SL, Kisseleva T, Brenner DA, et al. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol 2008;173:1617-27. [PubMed]
- Strieter RM, Gomperts BN, Keane MP. The role of CXC chemokines in pulmonary fibrosis. J Clin Invest 2007;117:549-56. [PubMed]
- Kisseleva T, Uchinami H, Feirt N, et al. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J Hepatol 2006;45:429-38. [PubMed]
- Ishida Y, Kimura A, Kondo T, et al. Essential roles of the CC chemokine ligand 3-CC chemokine receptor 5 axis in bleomycin-induced pulmonary fibrosis through regulation of macrophage and fibrocyte infiltration. Am J Pathol 2007;170:843-54. [PubMed]
- Sakai N, Wada T, Yokoyama H, et al. Secondary lymphoid tissue chemokine (SLC/CCL21)/CCR7 signaling regulates fibrocytes in renal fibrosis. Proc Natl Acad Sci U S A 2006;103:14098-103. [PubMed]
- Wada T, Sakai N, Matsushima K, et al. Fibrocytes: a new insight into kidney fibrosis. Kidney Int 2007;72:269-73. [PubMed]
- Moore BB, Kolodsick JE, Thannickal VJ, et al. CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am J Pathol 2005;166:675-84. [PubMed]
- Moore BB, Murray L, Das A, et al. The role of CCL12 in the recruitment of fibrocytes and lung fibrosis. Am J Respir Cell Mol Biol 2006;35:175-81. [PubMed]
- Phillips RJ, Burdick MD, Hong K, et al. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest 2004;114:438-46. [PubMed]
- Kisseleva T, von Köckritz-Blickwede M, Reichart D, et al. Fibrocyte-like cells recruited to the spleen support innate and adaptive immune responses to acute injury or infection. J Mol Med (Berl) 2011;89:997-1013. [PubMed]
- von Köckritz-Blickwede M, Goldmann O, et al. Phagocytosis-independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood 2008;111:3070-80. [PubMed]
- von Köckritz-Blickwede M, Nizet V. Innate immunity turned inside-out: antimicrobial defense by phagocyte extracellular traps. J Mol Med (Berl) 2009;87:775-83. [PubMed]
- Bellini A, Mattoli S. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Invest 2007;87:858-70. [PubMed]
- Mori L, Bellini A, Stacey MA, et al. Fibrocytes contribute to the myofibroblast population in wounded skin and originate from the bone marrow. Exp Cell Res 2005;304:81-90. [PubMed]
- Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 2002;418:41-9. [PubMed]
- Harting MT, Jimenez F, Cox CS Jr. Isolation of mesenchymal stem cells (MSCs) from green fluorescent protein positive (GFP+) transgenic rodents: the grass is not always green(er). Stem Cells Dev 2009;18:127-35. [PubMed]
- Forbes SJ, Russo FP, Rey V, et al. A significant proportion of myofibroblasts are of bone marrow origin in human liver fibrosis. Gastroenterology 2004;126:955-63. [PubMed]
- Kallis YN, Forbes SJ. The bone marrow and liver fibrosis: friend or foe? Gastroenterology 2009;137:1218-21. [PubMed]
- Russo FP, Alison MR, Bigger BW, et al. The bone marrow functionally contributes to liver fibrosis. Gastroenterology 2006;130:1807-21. [PubMed]
- Baertschiger RM, Serre-Beinier V, Morel P, et al. Fibrogenic potential of human multipotent mesenchymal stromal cells in injured liver. PLoS One 2009;4:e6657. [PubMed]
- Abdel Aziz MT, Atta HM, Mahfouz S, et al. Therapeutic potential of bone marrow-derived mesenchymal stem cells on experimental liver fibrosis. Clin Biochem 2007;40:893-9. [PubMed]
- Anjos-Afonso F, Bonnet D. Prospective identification and isolation of murine bone marrow derived multipotent mesenchymal progenitor cells. Best Pract Res Clin Haematol 2011;24:13-24. [PubMed]
- Banas A, Teratani T, Yamamoto Y. IFATS collection: in vivo therapeutic potential of human adipose tissue mesenchymal stem cells after transplantation into mice with liver injury. Stem Cells 2008;26:2705-12. [PubMed]
- Cho KA, Ju SY, Cho SJ, et al. Mesenchymal stem cells showed the highest potential for the regeneration of injured liver tissue compared with other subpopulations of the bone marrow. Cell Biol Int 2009;33:772-7. [PubMed]
- di Bonzo LV, Ferrero I, Cravanzola C, et al. Human mesenchymal stem cells as a two-edged sword in hepatic regenerative medicine: engraftment and hepatocyte differentiation versus profibrogenic potential. Gut 2008;57:223-31. [PubMed]
- Chesney J, Bacher M, Bender A, et al. The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in situ. Proc Natl Acad Sci U S A 1997;94:6307-12. [PubMed]
- Chesney J, Bucala R. Peripheral blood fibrocytes: novel fibroblast-like cells that present antigen and mediate tissue repair. Biochem Soc Trans 1997;25:520-4. [PubMed]
- Chesney J, Bucala R. Peripheral blood fibrocytes: mesenchymal precursor cells and the pathogenesis of fibrosis. Curr Rheumatol Rep 2000;2:501-5. [PubMed]
- Kisseleva T, Brenner DA. The phenotypic fate and functional role for bone marrow-derived stem cells in liver fibrosis. J Hepatol 2012;56:965-72. [PubMed]
- Galan A, Cowper SE, Bucala R. Nephrogenic systemic fibrosis (nephrogenic fibrosing dermopathy). Curr Opin Rheumatol 2006;18:614-7. [PubMed]
- Deng A, Martin DB, Spillane A, et al. Nephrogenic systemic fibrosis with a spectrum of clinical and histopathological presentation: a disorder of aberrant dermal remodeling. J Cutan Pathol 2010;37:204-10. [PubMed]
- Miyamoto J, Tanikawa A, Igarashi A, et al. Detection of iron deposition in dermal fibrocytes is a useful tool for histologic diagnosis of nephrogenic systemic fibrosis. Am J Dermatopathol 2011;33:271-6. [PubMed]
- Andersson-Sjöland A, de Alba CG, Nihlberg K, et al. Fibrocytes are a potential source of lung fibroblasts in idiopathic pulmonary fibrosis. Int J Biochem Cell Biol 2008;40:2129-40. [PubMed]
- Gomperts BN, Strieter RM. Fibrocytes in lung disease. J Leukoc Biol 2007;82:449-56. [PubMed]
- Herzog EL, Bucala R. Fibrocytes in health and disease. Exp Hematol 2010;38:548-56. [PubMed]
- Kleaveland KR, Moore BB, Kim KK. Paracrine functions of fibrocytes to promote lung fibrosis. Expert Rev Respir Med 2014;8:163-72. [PubMed]
- Moeller A, Gilpin SE, Ask K, et al. Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2009;179:588-94. [PubMed]
- Schmidt M, Sun G, Stacey MA, et al. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol 2003;171:380-9. [PubMed]
- Yang L, Scott PG, Giuffre J, et al. Peripheral blood fibrocytes from burn patients: identification and quantification of fibrocytes in adherent cells cultured from peripheral blood mononuclear cells. Lab Invest 2002;82:1183-92. [PubMed]
- Sakai N, Furuichi K, Shinozaki Y, et al. Fibrocytes are involved in the pathogenesis of human chronic kidney disease. Hum Pathol 2010;41:672-8. [PubMed]
- Sakai N, Wada T, Matsushima K, et al. The renin-angiotensin system contributes to renal fibrosis through regulation of fibrocytes. J Hypertens 2008;26:780-90. [PubMed]
- Nunnari G, Vancheri C, Gilli E, et al. Circulating fibrocytes as a marker of liver fibrosis in chronic hepatitis C. Front Biosci (Elite Ed) 2010;2:1241-5. [PubMed]
- Sahebally SM, Burke JP, Chang KH, et al. Circulating fibrocytes and Crohn’s disease. Br J Surg 2013;100:1549-56. [PubMed]
- Mathai SK, Gulati M, Peng X, et al. Circulating monocytes from systemic sclerosis patients with interstitial lung disease show an enhanced profibrotic phenotype. Lab Invest 2010;90:812-23. [PubMed]
- Hong KM, Burdick MD, Phillips RJ, et al. Characterization of human fibrocytes as circulating adipocyte progenitors and the formation of human adipose tissue in SCID mice. FASEB J 2005;19:2029-31. [PubMed]
- Kisseleva T, Uchinami H, Feirt N, et al. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J Hepatol 2006;45:429-38. [PubMed]
- Scholten D, Reichart D, Paik YH, et al. Migration of fibrocytes in fibrogenic liver injury. Am J Pathol 2011;179:189-98. [PubMed]
- Mehrad B, Burdick MD, Strieter RM. Fibrocyte CXCR4 regulation as a therapeutic target in pulmonary fibrosis. Int J Biochem Cell Biol 2009;41:1708-18. [PubMed]
- Keeley EC, Mehrad B, Strieter RM. The role of circulating mesenchymal progenitor cells (fibrocytes) in the pathogenesis of fibrotic disorders. Thromb Haemost 2009;101:613-8. [PubMed]
- Seki E, De Minicis S, Gwak GY, et al. CCR1 and CCR5 promote hepatic fibrosis in mice. J Clin Invest 2009;119:1858-70. [PubMed]
- Seki E, de Minicis S, Inokuchi S, et al. CCR2 promotes hepatic fibrosis in mice. Hepatology 2009;50:185-97. [PubMed]
- Hong KM, Belperio JA, Keane MP, et al. Differentiation of human circulating fibrocytes as mediated by transforming growth factor-beta and peroxisome proliferator-activated receptor gamma. J Biol Chem 2007;282:22910-20. [PubMed]
- Balmelli C, Alves MP, Steiner E, et al. Responsiveness of fibrocytes to toll-like receptor danger signals. Immunobiology 2007;212:693-9. [PubMed]
- Strieter RM, Keeley EC, Hughes MA, et al. The role of circulating mesenchymal progenitor cells (fibrocytes) in the pathogenesis of pulmonary fibrosis. J Leukoc Biol 2009;86:1111-8. [PubMed]
- Reilkoff RA, Bucala R, Herzog EL. Fibrocytes: emerging effector cells in chronic inflammation. Nat Rev Immunol 2011;11:427-35. [PubMed]
- Ekert JE, Murray LA, Das AM, et al. Chemokine (C-C motif) ligand 2 mediates direct and indirect fibrotic responses in human and murine cultured fibrocytes. Fibrogenesis Tissue Repair 2011;4:23. [PubMed]
- Garibaldi BT, D’Alessio FR, Mock JR, et al. Regulatory T cells reduce acute lung injury fibroproliferation by decreasing fibrocyte recruitment. Am J Respir Cell Mol Biol 2013;48:35-43. [PubMed]
- Niedermeier M, Reich B, Rodriguez Gomez M, et al. CD4+ T cells control the differentiation of Gr1+ monocytes into fibrocytes. Proc Natl Acad Sci U S A 2009;106:17892-7. [PubMed]
- Chesney J, Metz C, Stavitsky AB, et al. Regulated production of type I collagen and inflammatory cytokines by peripheral blood fibrocytes. J Immunol 1998;160:419-25. [PubMed]
- Shao DD, Suresh R, Vakil V, et al. Pivotal Advance: Th-1 cytokines inhibit, and Th-2 cytokines promote fibrocyte differentiation. J Leukoc Biol 2008;83:1323-33. [PubMed]
- Pilling D, Vakil V, Gomer RH. Improved serum-free culture conditions for the differentiation of human and murine fibrocytes. J Immunol Methods 2009;351:62-70. [PubMed]
- Pilling D, Fan T, Huang D, et al. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS One 2009;4:e7475. [PubMed]
- Murray LA, Rosada R, Moreira AP, et al. Serum amyloid P therapeutically attenuates murine bleomycin-induced pulmonary fibrosis via its effects on macrophages. PLoS One 2010;5:e9683. [PubMed]
- Suga H, Rennert RC, Rodrigues M, et al. Tracking the elusive fibrocyte: identification and characterization of collagen-producing hematopoietic lineage cells during murine wound healing. Stem Cells 2014;32:1347-60. [PubMed]
- Higashiyama R, Moro T, Nakao S, et al. Negligible contribution of bone marrow-derived cells to collagen production during hepatic fibrogenesis in mice. Gastroenterology 2009;137:1459-66.e1.
- Kisseleva T, von Köckritz-Blickwede M, Reichart D, et al. Fibrocyte-like cells recruited to the spleen support innate and adaptive immune responses to acute injury or infection. J Mol Med (Berl) 2011;89:997-1013. [PubMed]
- Scholten D, Osterreicher CH, Scholten A, et al. Genetic labeling does not detect epithelial-to-mesenchymal transition of cholangiocytes in liver fibrosis in mice. Gastroenterology 2010;139:987-98. [PubMed]
- Scholten D, Reichart D, Paik YH, et al. Migration of fibrocytes in fibrogenic liver injury. Am J Pathol 2011;179:189-98. [PubMed]
- Niedermeier M, Reich B, Rodriguez Gomez M, et al. CD4+ T cells control the differentiation of Gr1+ monocytes into fibrocytes. Proc Natl Acad Sci U S A 2009;106:17892-7. [PubMed]
- Crawford JR, Pilling D, Gomer RH. Improved serum-free culture conditions for spleen-derived murine fibrocytes. J Immunol Methods 2010;363:9-20. [PubMed]
- Curnow SJ, Fairclough M, Schmutz C, et al. Distinct types of fibrocyte can differentiate from mononuclear cells in the presence and absence of serum. PLoS One 2010;5:e9730. [PubMed]
- Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science 2004;303:1532-5. [PubMed]
- Yousefi S, Gold JA, Andina N, et al. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med 2008;14:949-53. [PubMed]
- Chow OA, von Köckritz-Blickwede M, Bright AT, et al. Statins enhance formation of phagocyte extracellular traps. Cell Host Microbe 2010;8:445-54. [PubMed]
- Brinkmann V, Zychlinsky A. Beneficial suicide: why neutrophils die to make NETs. Nat Rev Microbiol 2007;5:577-82. [PubMed]
- Kessenbrock K, Krumbholz M, Schönermarck U, et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med 2009;15:623-5. [PubMed]
- Balmelli C, Ruggli N, McCullough K, et al. Fibrocytes are potent stimulators of anti-virus cytotoxic T cells. J Leukoc Biol 2005;77:923-33. [PubMed]
- Baker K, Rath T, Pyzik M, et al. The Role of FcRn in Antigen Presentation. Front Immunol 2014;5:408. [PubMed]
- Grage-Griebenow E, Flad HD, Ernst M. Heterogeneity of human peripheral blood monocyte subsets. J Leukoc Biol 2001;69:11-20. [PubMed]
- Passlick B, Flieger D, Ziegler-Heitbrock HW. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood 1989;74:2527-34. [PubMed]
- Castaño AP, Lin SL, Surowy T, et al. Serum amyloid P inhibits fibrosis through Fc gamma R-dependent monocyte-macrophage regulation in vivo. Sci Transl Med 2009;1:5ra13.
- Pilling D, Buckley CD, Salmon M, et al. Inhibition of fibrocyte differentiation by serum amyloid P. J Immunol 2003;171:5537-46. [PubMed]
- Mold C, Gresham HD, Du Clos TW. Serum amyloid P component and C-reactive protein mediate phagocytosis through murine Fc gamma Rs. J Immunol 2001;166:1200-5. [PubMed]
- Mantovani A, Sozzani S, Locati M, et al. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 2002;23:549-55. [PubMed]
- Cathcart ES, Wollheim FA, Cohen AS. Plasma protein constituents of amyloid fibrils. J Immunol 1967;99:376-85. [PubMed]
- Ashton AW, Boehm MK, Gallimore JR, et al. Pentameric and decameric structures in solution of serum amyloid P component by X-ray and neutron scattering and molecular modelling analyses. J Mol Biol 1997;272:408-22. [PubMed]
- Bijl M, Bootsma H, Van Der Geld Y, et al. Serum amyloid P component levels are not decreased in patients with systemic lupus erythematosus and do not rise during an acute phase reaction. Ann Rheum Dis 2004;63:831-5. [PubMed]
- Pilling D, Tucker NM, Gomer RH. Aggregated IgG inhibits the differentiation of human fibrocytes. J Leukoc Biol 2006;79:1242-51. [PubMed]
- Cox N, Pilling D, Gomer RH. Serum amyloid P: a systemic regulator of the innate immune response. J Leukoc Biol 2014;96:739-43. [PubMed]
- Murray LA, Rosada R, Moreira AP, et al. Serum amyloid P therapeutically attenuates murine bleomycin-induced pulmonary fibrosis via its effects on macrophages. PLoS One 2010;5:e9683. [PubMed]
- Pilling D, Roife D, Wang M, et al. Reduction of bleomycin-induced pulmonary fibrosis by serum amyloid P. J Immunol 2007;179:4035-44. [PubMed]
- Iwata Y, Yoshizaki A, Ogawa F, et al. Increased serum pentraxin 3 in patients with systemic sclerosis. J Rheumatol 2009;36:976-83. [PubMed]
- Naik-Mathuria B, Pilling D, Crawford JR, et al. Serum amyloid P inhibits dermal wound healing. Wound Repair Regen 2008;16:266-73. [PubMed]
- Moalli F, Paroni M, Véliz Rodriguez T, et al. The therapeutic potential of the humoral pattern recognition molecule PTX3 in chronic lung infection caused by Pseudomonas aeruginosa. J Immunol 2011;186:5425-34. [PubMed]
- Chiarini M, Sabelli C, Melotti P, et al. PTX3 genetic variations affect the risk of Pseudomonas aeruginosa airway colonization in cystic fibrosis patients. Genes Immun 2010;11:665-70. [PubMed]
- Duffield JS, Forbes SJ, Constandinou CM, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest 2005;115:56-65. [PubMed]
- Sazuka S, Katsuno T, Nakagawa T, et al. Fibrocytes are involved in inflammation as well as fibrosis in the pathogenesis of Crohn’s disease. Dig Dis Sci 2014;59:760-8. [PubMed]
- Friedenstein AJ, Petrakova KV, Kurolesova AI, et al. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 1968;6:230-47. [PubMed]
- Shiota M, Heike T, Haruyama M, et al. Isolation and characterization of bone marrow-derived mesenchymal progenitor cells with myogenic and neuronal properties. Exp Cell Res 2007;313:1008-23. [PubMed]
- Ferrari G, Cusella-De Angelis G, Coletta M, et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science 1998;279:1528-30. [PubMed]
- Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci U S A 1999;96:10711-6. [PubMed]
- Krampera M, Glennie S, Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 2003;101:3722-9. [PubMed]


