Pancreatic cancer surgery and nutrition management: a review of the current literature
Introduction
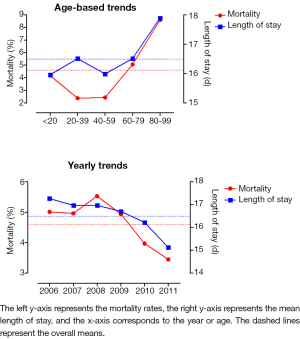
Pancreatic cancer is the 4th leading cause of cancer death in the United States, despite being the 12th most incident cancer. Complete surgical resection is the only therapy with the possibility of long-term survival. The first large series of 41 patients undergoing pancreaticoduodenectomy (PD), or Whipple procedure, was reported in 1941 (1). The mortality rate was 29%. Most of the improved survival achieved over the past 3 decades has been related to improved perioperative management, and earlier recognition and treatment of post-operative morbidity. Mortality rates are currently <5% at high-volume pancreatic surgery centers (2,3). In fact, mortality rates have remained relatively low in the United States over the last decade (Figure 1) (4).
Despite significant improvement in mortality, morbidity remains high, ranging from 30-60% in some reports (3,5,6). Risk stratification and decreasing morbidity are essential to improving outcomes following a procedure with such high morbidity at baseline. The most serious complication remains development of a pancreatic fistula (PF), which can occur in 20% of patients (3,6,7). Sequelae of PFs include deep-space surgical site infections (SSIs) and sepsis, which can be associated with mortality rates of 40% (8). In a series of 132 patients undergoing pancreatic surgery, Sierzega et al. demonstrated an association between malnutrition and PF (9). On multivariable analyses, the only factor significantly predicting PF was a nutritional risk index (NRI) score of 100 or less (OR =8.12, 95% CI: 1.06-22.30; P<0.05). Schnelldorfer et al. found that patients with a low serum albumin undergoing surgery for chronic pancreatitis were at greater risk of developing a PF (P=0.04) (10). With a post-operative 20-25% 5-year survival, any time lost to morbidity that can be prevented needs to be further understood and addressed.
Malnutrition, a medical condition caused by improper or insufficient diet, has been determined to be an independent risk factor for morbidity and mortality in patients undergoing surgical procedures. This includes increased incidence of superficial and deep SSIs, sepsis, impaired wound healing, failure of ventilator weaning, pneumonia, renal insufficiency, cardiac and neurologic events, re-admission, length of stay and overall costs (11-15). This leads to a vicious cycle, as complications are detrimental to the nutritional state of the patient.
The operative field for pancreatectomy is at the intersection of the digestive system. The flow of food, hormonal stimulation, enzyme release and digestive vasculature are affected by the location of the malignancy and the operative reconstruction. Patients with pancreatic carcinoma present with a high frequency of malnutrition-related signs and symptoms at the time of diagnosis, including weight loss (85%), anorexia (83%), abdominal pain (79%), epigastric pain (71%), nausea (51%), diarrhea (44%), vomiting (33%), and steatorrhea (25%) (16). A moderate to severe risk of malnutrition was identified in 52-88% of patients who underwent pancreatic resection for cancer (13). Yet there is scant data to optimally nourish patients in the perioperative period despite the recognized malnourished state and associated increased morbidity and mortality.
Malnutrition has been documented to be an independent risk factor in surgical outcomes for nearly 80 years, thus identifying patients at risk prior to surgery may be critical to improving outcomes (13,17). Patients should be screened for nutritional risk, and nutritional intervention should be provided early in treatment to optimize outcomes. Early identification and intervention has been shown to reduce morbidity, length of stay, and admission costs in hospitalized patients (17-19). The following is a review of available literature regarding pancreatic cancer surgery and perioperative nutritional considerations and strategies.
Methods
A systematic search was performed using PubMed for studies published through May 26, 2014. Search terms used were ‘pylorous preserving PD or pancreatic resection or pancreatectomy or Whipple or pancreatic surgery or duodenal preserving pancreatic head resection’ and ‘nutrition or feeding or nasogastric or nasojejunal or gastrojejunostomy or jejunostomy’, restricted to title, abstract or keywords. We sought articles with level I evidence whenever possible; however, the majority of the literature was comprised of level II or greater evidence. Systematic reviews, meta-analyses, randomized and observational cohort studies were included. Opinion papers, case reports, and animal studies were excluded for this review. Perioperative, as used in this manuscript, encompasses the period from diagnosis, through surgery, to full recovery with oral intake. Management of acute and chronic pancreatitis was not included.
Preoperative nutrition assessment
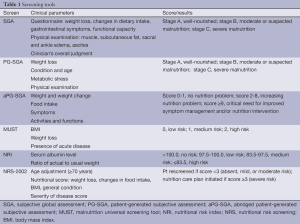
In general, malignancies predispose patients to preoperative malnutrition. Proper screening for malnutrition can help identify patients at increased risk for perioperative morbidity. Unfortunately, the terminology surrounding malnutrition remains quite confusing. Manifestations of disease-related catabolism are often indistinguishable from those related to starvation, and patients with malnutrition may not be well fed calorically. That is, patients may lack a diet filled with nutrients and protein despite being capable of efficiently metabolizing the available sources of nutrition. Various screening tools have been developed and validated for identifying patients at risk of malnutrition, including the subjective global assessment (SGA), malnutrition universal screening tool (MUST), and NRI (20) (Table 1). These tools, in conjunction with certain anthropometric measurements, such as body mass index (BMI) and laboratory markers of nutrition, such as albumin and prealbumin, can help guide preoperative strategies to improve patient nutrition. Though significant weight loss is considered a reliable indicator, malnutrition is far more complex. Even patients with a high BMI may be at considerable risk of malnutrition (13,21,22).
Full table
The SGA requires a physical examination by a health professional (21). Therefore, time constraints and ease of use may be barriers. The patient-generated SGA (PG-SGA) was developed for the oncology population and includes questions to be filled out by the patient in addition to the physical examination and has been shown to effectively identify malnutrition (22,23). Recently, the abridged PG-SGA (aPG-SGA) was found to be an effective tool at identifying cancer cachexia and predicting outcomes including risk for chemotherapy intolerance and life expectancy (24). The MUST and NRS-2002 have been validated for use in hospitalized patients with high sensitivity and specificity for predicting postoperative morbidity (23,25-28). The NRI failed to detect surgical or oncology patients at high risk for malnutrition (25,26) but was found to be an independent factor in predicting SSI after PD (27). Using ≥5% weight loss during the 6 months prior to surgery was found to be as reliable as SGA, MUST, and NRS-2002, whereas low BMI alone was shown to be an unreliable measure of malnutrition (23,25,26). Using BMI as a single measure to assess malnutrition risk amongst patients with pancreatic cancer would overlook as many as 21-24% of patients who were classified as overweight or obese by the World Health Organization, as high BMI may reflect an excess of certain nutrients or nutrients in wrong proportions (29).
Only one study has compared these measures to evaluate the prevalence and effect of malnutrition on postoperative morbidity for patients undergoing resection of pancreatic cancer (13). On its own, weight loss of ≥5% preadmission over the preceding three to six months was related to an increased risk of SSI and increased length of stay. The MUST and NRI showed excellent agreement with regards to overall morbidity, SSI rate, and length of hospital stay, while MUST and SGA had excellent agreement regarding SSI rate (13). Nevertheless, this was a retrospective review.
Preoperative serum markers
Albumin is an acute phase protein which decreases during periods of inflammation, trauma, and injury. It has long been known that albumin is not reflective of the adequacy of a patient’s intake (30). However, hypoalbuminemia is strongly associated with poor postoperative outcomes, such as mortality and infection following gastrointestinal surgery (31). Amongst patients undergoing resection for pancreatic adenocarcinoma (n=268), preoperative hypoalbuminemia (<4 g/dL) was associated with an increase in postoperative complications (40.3% versus 25.5%; P<0.05), as cited in the retrospective review by Kanda and colleagues (17).
C-reactive protein (CRP) is an acute phase protein which also increases during periods of inflammation, trauma, and injury. Elevated preoperative CRP have been associated with a worse prognosis for various cancers (32,33). Patients with an elevated preoperative CRP (>10 mg/L) had a significantly shorter survival (8.3 versus 18.2 months; P<0.05) than patients with lower CRP levels (≤10 mg/L) in one series of 65 patients (34). The majority of this data is based on retrospective reviews.
It is clear that systemic inflammation is associated with increased weight loss, functional decline, loss of lean tissue, and overall poor prognosis (35). The Glasgow prognostic score (GPS) measures both albumin and CRP. It has been shown to be a reliable prognostic indicator for survival in various cancers, independent of tumor stage, including patients undergoing palliative resection for advanced pancreatic cancer (36). The GPS (Table 2) may be useful in identifying patients at high risk for malnutrition.
Preoperative counseling
The Enhanced Recovery After Surgery (ERAS) Society has evaluated various preoperative and intraoperative measures that may influence postoperative outcomes following pancreatic surgery (37). One of those preoperative measures was the effect of proper preoperative counseling, including meeting with a specialist in nutrition. Although evidence specific to pancreatic surgery is lacking, there is strong support for this approach. The use of preoperative multidisciplinary counseling has been used with success in other surgical specialties including colorectal, bariatric and transplant surgery (38,39).
Perioperative nutrition
Malnourishment before and prolonged fasting after major abdominal surgery are significant risk factors for adverse outcomes (40-42). The role of perioperative nutrition in malnourished patients has been studied to some extent in other forms of gastrointestinal malignancies. In a prospective randomized controlled trial by Wu and colleagues [2006] 468 patients with moderate to severe malnutrition (as defined by the clinician) with gastric, colon, or rectal cancer were randomly divided to receive a standard oral nutrition (control group) preoperatively or parenteral or enteral nutrition for 8 to 10 days preoperatively (study group) (43). The mortality and complication rates were significantly lower in the study group (2.1% vs. 6.0%, P=0.003 and 18.3% vs. 33.5%, P=0.012, respectively). The most frequent complication in all groups was infection related to debilitation and/or immobility. Septic complications were not significantly different between the two groups, nor between those patients receiving parenteral versus enteral nutrition (P>0.05). There remains considerable debate on how best to nourish patients prior to pancreatic surgery, as well as in the postoperative period. There does not appear to be benefits to providing supplemental nutrition to well-nourished patients in the pre-operative period. And in a small randomized controlled trial of well-nourished patients undergoing PD or esophagectomy enterally fed immediately post-operatively versus initiation on post-operative day 6, the early fed group unexpectedly had a greater decrement in respiratory mechanics as measured by vital capacity and FEV1. Other measurements of strength, fatigue, weight and anastomotic leak were not significantly different between the two groups, and the authors concluded that immediate postoperative enteral feeding should not be used in well-nourished patients routinely (44).
Oral feeding
Various reports have studied the efficacy of early oral feeding strategies following pancreatic surgery. According to the ERAS Society recommendations, routine use of preoperative enteral nutrition is not indicated (37). However, there is low-level evidence suggesting preoperative supplemental nutrition may be indicated in the malnourished patient. The European Society for Clinical Nutrition and Metabolism (ESPEN) more strongly supports preoperative nutritional support for 10-14 days in patients at severe nutritional risk, even if surgery needs to be delayed. ESPEN defined severe risk by the presence of at least one of the following criteria: weight loss >10-15% within 6 months, BMI <18.5 kg/m2, SGA grade C, serum albumin <3 g/dL (45).
Routine use of postoperative enteral tube feeding is not indicated and patients should be started on a normal, oral diet, with a gradual increase over 3 to 4 days. There is soft evidence referenced in ERAS recommendations that fast-track oral feeding strategies result in less delayed gastric emptying (DGE) than normal oral feeding strategies. ESPEN guidelines also support early initiation of normal food within 24 hours after major gastrointestinal surgery. Again ESPEN more strongly argues for simultaneous enteral nutrition supplied beyond anastomoses in patients that cannot achieve >60% of their nutritional needs within 10 days and/or with obvious under nutrition at the time of surgery (45).
The discrepancy between ERAS and ESPEN guidelines recognizes that most patients are incapable of attaining their nutritional goals per os in the post-operative period. In Bozzetti’s letter [2013], the discrepancies between planned feeding schedules and intake outcomes are pointed out in studies of patients undergoing pancreatectomy (46-53). In response, Lassen and associates point out that some of the literature supporting the ESPEN approach also suffers qualitatively and that enteral tubes are not risk free (54).
A recent ERAS study of 115 patients undergoing PD by Braga and associates aimed to start liquids on post-operative day 1 and solids on post-operative day 2 in the ERAS group, versus post-operative day 3 and post-operative day 4, respectively, in the historical control group. These objectives were achieved in 55% of patients for oral liquid targets and 53% for solid food targets. Low compliance with ERAS targets was related to rate and severity of complications. For example, of the 60 patients with poor compliance to early oral feeding, nearly 72% had post-operative complications (55).
Oral feeding strategies remain the preferred modality following pancreatic surgery. In a meta-analysis by Gerritsen and colleagues [2013], mean length of stay was shortest in the oral diet (15 days) and gastrojejunostomy (GJT) (15 days) groups compared to the jejunostomy (JT) (19 days), parenteral nutrition (PN) (20 days), and nasojejunal tube (NJT) (25 days) groups (56). Even when assessing the efficacy of early fast-track feeding strategies, various reports failed to show an improvement in length of stay (57-59). According to Gerritsen and colleagues [2013], the mean time to resumption of a normal diet was fastest in the oral group (6 days), compared to the NJT (8 days), PN (11 days), JT (12 days), and GJT (14 days) groups (56). An estimated 49.4% of patients experienced a complication in the oral feeding group, which was only higher than the JT group (43.8%). The nature of the complications was not included in the report. Mortality rates ranged from 1.8% in the NJT group to 4.4% in the oral group, to 5.4% in the PN group. The incidence of DGE and PF were 14.1% and 7.7%, respectively, in the oral feeding group. Again it should be noted that this was an observational analysis and not a prospective study. Martignoni et al. found no difference in mean reported weight loss during the hospital stay when comparing oral feeding to enteral nutrition groups (3.8 vs. 4.4 kg; P>0.05) (58). However, this too was a retrospective study.
Allowing patients to eat at will postoperatively has been supported by various surgical subspecialties, including colorectal and bariatric surgery (60,61). In a prospective randomized controlled trial from multiple institutions, Lassen and colleagues randomized patients to enteral tube feeding (needle catheter jejunostomy tube) (N=227) or food at will (N=220) following upper gastrointestinal surgery, (e.g., gastrectomies, pancreatic surgery, hepatic resections, biliary surgery, esophagectomies) (62). A total of 18.4% (n=82) of subjects underwent a Whipple. There were significantly less major complications in the food at will group (100 in 220 patients) compared to the enteral tube feeding group (165 in 227 patients) (P=0.01). There was no significant difference in reoperation rate (P=0.50), thirty-day mortality (P=0.83), or total mortality within the trial period (P=0.36) between the two groups. Adjusting for presence or lack of an upper gastrointestinal anastomosis did not result in any significant difference between the two groups, including anastomotic leak rate, major infectious complication or percent of patients with a major complication. Mean time to flatus was significantly shorter in the food at will group (2.6 vs. 3 days, P=0.01); time to first bowel movement was not significantly different (P=0.11). Mean length of stay was significantly shorter in the food at will group (13.5 vs. 16.7 days, P=0.046). The overall enteral feeding tube complication rate was 7.2% and the reoperation rate caused by the catheter was 1.3%.
Parenteral nutrition
PN provides a means of nourishment for patients in whom oral or enteral nutrition is not possible or practical. The appropriate selection of patients for use of PN is important because it causes more harm than benefit in patients who can tolerate enteral nutrition or who are not malnourished. According to ASPEN and ESPEN guidelines, PN is generally regarded to be appropriate and beneficial in the post-surgical period in undernourished patients in whom enteral nutrition is not feasible or tolerated within 7-10 days of their procedure. PN is associated with an increased risk of bloodstream infection (especially fungemia), independent of and in addition to the risk of central venous catheterization alone, as well as decreased likelihood of earlier live discharge from the intensive care unit postoperatively (63-67). PN is also associated with the development of metabolic complications, including refeeding syndrome, hyperglycemia, and serum electrolyte abnormalities. It is important to recognize that some of the historical limitations of PN were related to inappropriate formulations heavy in carbohydrate calories, high volume preparations, poor concomitant glycemic control and hyperalimentation. PN can be a life saving form of nutritional supplementation when appropriately used and formulated to meet the needs of individual patients, alone or in combination with enteral or per os nutrition (64).
Authors have attempted to demonstrate a role for routine PN in post PD patients. Despite early enthusiasm for PN, oral nutrition has consistently been shown to be safer and more effective than PN with respect to occurrence of post-operative complications (including infection, PF and DGE) and length of stay (57,68). In a prospective randomized controlled trial by Klek and colleagues [2011], 167 malnourished cancer patients were randomly assigned to receive either enteral or parenteral and standard or immunomodulating nutrition for 14 days before undergoing surgery to assess the effect on postoperative complications (69). Malnutrition was defined by the ESPEN criteria presented earlier (45). The authors found that immunomodulating enteral feeds in malnourished patients significantly decreased overall morbidity (P=0.01), infectious complications (P=0.04), mortality (P=0.03), and length of stay (P=0.006) compared to standard enteral feeding. Immunomodulation made no significant difference in the PN arm with respect to morbidity, mortality, or length of stay (P>0.05). In cases of prolonged gastrointestinal dysfunction where enteral feeding strategies are not possible, PN should be given until caloric requirements are met per os.
PN has also been suggested as a potential tool in the conservative management of PF; however, other feeding modalities have proven more effective. Klek et al. [2011] performed a prospective randomized controlled trial of 78 patients with PFs randomized to either EN or PN (70). At 30 days, the PF closure rate was 60% in the EN group compared to 37% in the PN group (P=0.04). The median time to closure in the EN group was 27 days, while the median time was not reached at the conclusion of the study for the PN group (P=0.047). The only two factors associated with PF closure were EN [OR =6.136, 95% confidence interval (CI): 1.204-41.623; P=0.04] and initial fistula output ≤200 cc/day (OR =12.701; 95% CI: 9.102-47.241; P<0.001). It should be noted that DGE can be well managed with distal feeding tubes, so PN should not be necessary in these patients.
Enteral nutrition
EN via a tube passed through the nose or abdominal wall provides a means of supplementing per os intake or ensuring adequate nutrient intake when per os feeding is not practical, with fewer severe risks than PN. When compared to PN in the general surgical literature, EN has been shown to lead to reduced infections, decreased mortality, shorter length of stay, and to be more cost effective (71-73). In the absence of gastrointestinal dysfunction, the evidence supports the use of EN over PN when per os nutrition is not possible. However, many questions remain with respect to timing, site of tube feeding, oral vs. tube feeds, and type of formula. This decision-making process is further complicated by the relatively common occurrence of DGE post-operatively in the pancreatic surgery cohort. The complexity of these decisions requires PD patients be cared for by a multidisciplinary team, including nutrition professionals.
More recent publications endorse the benefit of different enteral nutrition routes. Zhu et al. demonstrated the superiority of NJT to JT with respect to complications and length of hospital stay in a randomized, controlled clinical study (74). Gerritsen and colleagues [2012] after their systematic analyses reported their own experience with NJ, JT and PN (75). In this review, NJT feeding (44 patients) was compared to JT feeding (48 patients) and PN (37 patients). There was no difference in time to resumption of oral intake between NJT feeding (median 13 days), JT feeding (16 days) and PN (14 days) (P=0.15). Abu-Hilal et al. found that NJT feeds following pancreatic surgery led to resumption of a normal diet faster than GJT or JT feeds (median 10 vs. 14 vs. 14 days, respectively; P=0.02) (76). In the meta-analysis by Gerritsen et al. [2013], there was no difference in length of stay between the three groups (P=0.35). The time to resumption of a normal diet was longest in the GJT group (mean 14 days), 12 days in the JT group, and shortest in the oral diet group (mean 6 days) (56).
Scaife and colleagues attempted to retrospectively identify risk factors that predict the need for enteral feeding tubes, and found a number of factors that may help predict those that will require assistance post-operatively (77). Patients were categorized according to the presence or absence of the following ten NSQIP preoperative risk factors, including preoperative dependent functional status; presence of chronic obstructive pulmonary disease (COPD); advanced age; male gender; elevated creatinine; leukocytosis; steroid use; bleeding disorders; hypoalbuminemia; and increased BMI. The most important single predictor in terms of feeding tube need was age ≥80 years (P=0.035). There were no complications related to feeding tube placement, regardless of timing of placement. Of the 56 feeding tube placed intraoperatively, 16.1% required replacement for clogging, inadvertent removal, and premature removal. They also estimated a benefit in terms of cost by prospectively implementing a strategy of inserting feeding tubes at the time of operation, dependent on the presence of these pre-operative risk factors. In a theoretical population of 100 patients, there was a cost savings of US $4,050.
In the majority of cases patients should be allowed to eat at will. Enteral feeding strategies, while superior to PN, should only be employed selectively and tubes should not be routinely inserted. PN should be utilized only when other forms of enteral nutrition are not possible. Following these strategies should decrease length of stay by allowing quicker resumption of per os nutrition, which may additionally minimize costs.
Perioperative enteral tubes
The role of enteral tubes has been highly debated and fairly surgeon specific. The specific evidence favoring an optimal decompression and feeding strategy following pancreatic surgery is lacking. Table 3 compares four different feeding modalities. We describe the role of perioperative nasogastric tube decompression as well as perioperative feeding enteral tubes following pancreatic surgery.

Full table
Draining (sump) nasogastric tubes
Placement of draining NGT to prevent gastric distension, emesis, anastomotic leaks, and decrease time to return of bowel function following pancreatic cancer surgery has been considered standard practice (78). Recent data, suggests that NGT decompression may be unnecessary following pancreatic surgery. In a retrospective cohort study Fisher et al. described a series of 100 consecutive patients undergoing pancreatic surgery, with 50 patients having the NGT removed once patients demonstrated adequate bowel function (NGT group) and 50 patients having the NGT removed immediately postoperatively (no NGT group) (79). The mortality and morbidity rates were similar between the NGT and No NGT groups (0% vs. 2%, respectively; P=1.0 and 44% vs. 44%, respectively; P=1.0), as was the time to return of bowel function (median 5 vs. 5 days, respectively; P=0.81). The incidence of biliary anastomotic leaks was 0% in both groups. The PF rates were 6% in the NGT group and 10% in the no NGT group (P=0.72). Furthermore, length of stay was not significantly different between the two groups (median 7 in both groups; P=0.30). There were no complications from NGT insertion postoperatively (2 in the NGT group vs. 4 in the no NGT group; P=0.68).
In another observational cohort study of 250 patients [125 patients in each group (routine NGT & selective NGT)] undergoing PD, the authors concluded routine use of NGTs may be unnecessary (80). Selective NGT placement referred to those tubes placed when clinically indicated, such as for prolonged endotracheal intubation. The overall morbidity was not significantly different between the routine NGT and selective NGT groups (81.6% vs. 80.8%, respectively; P=NS). On multivariate analysis, routine use of NGT was an independent risk factor for DGE [hazard ratio (HR) =8.56; P=0.03]. Moreover, overall length of stay was significantly shorter in the selective NGT group compared to the routine NGT group (median 6 vs. 7 days, respectively; P<0.0001). Finally, return of bowel function was significantly shorter in the Selective NGT group (median 4 vs. 5 days, respectively; P<0.0001).
Gastrojejunostomy tubes
GJT are routinely placed at some institutions following pancreatic surgery. The benefits include the ability to feed distal to the area of resection, while also maintaining the ability to vent the stomach through the gastrostomy port. As mentioned, the incidence of DGE ranges from 6% to 45% following any pancreatic surgery (56,81,82). In a study by Mack and colleagues, 36 patients were randomized to GJT placement (20 patients) or standard NGT placement (16 patients) following PD to assess the impact on development of DGE (59). The overall complication rate was not significantly different between the GJT and NGT groups (20% vs. 25%, respectively; P=NS). The incidence of gastroparesis was 0% in the GJT group vs. 25% in the NGT group (P=0.03). Moreover, the duration of gastric decompression was significantly shorter in the GJT group compared to the NGT group (mean 5.3 vs. 9.5 days, respectively; P=0.02). Length of stay was significantly shorter in the GJT group (median 11.5 vs. 14 days, respectively; P=0.01). Finally, overall hospital charges were significantly less in the GJT group compared to the NGT group (mean US $52,589 vs. $82,151, respectively; P=0.04).
Though randomized, this study was limited by non-standardization of gastric decompression, route and type of nutritional supplementation in the control groups
Nasojejunal tubes
NJT feeding emerged as a feeding modality as a result of perceived complications related to JT and PN. Gerritsen and colleagues [2012] retrospectively reviewed a series of 129 patients undergoing PD over 10 years (75). Overall morbidity rates were not significantly different between the 3 groups (NJT 84% vs. JT 92% vs. PN 92%, respectively; P=0.49). However, tube related morbidity was highest in the NJT group (41%) compared to the JT (23%) and PN (16%) groups (P=0.03). The most frequent tube-related complication in the NJT group was dislodgement (34%), while the JT was the only group requiring return to the operating room for complications related to the tube (6%). There was a trend toward significance in tube-related morbidity between the NJT and JT groups (P=0.06). There was one tube-related mortality in the JT group, compared to none in the NJT and PN groups; however, this was not statistically significant (P=1.0). There was no difference in the rate of DGE in NJT (34%), JT (50%), and PN (40%) groups (P=0.30). Moreover, there was no difference in length of stay between NJT (median 17 days), JT (19 days), and PN (16 days) groups (P=0.83). The authors concluded that none of the feeding strategies was superior to the other.
Jejunostomy tubes
JT feeding has historically been employed in pancreatic cancer surgery to initiate early enteral nutrition in a relatively malnourished patient. Several studies have evaluated the efficacy and complications associated with JT placement and feeding. In the study by Gerritsen et al. [2012], the most serious complications occurred in the JT group, including four tube-related relaparotomies and one tube-related mortality (75). Complications specific to JTs included mechanical bowel obstructions and leakage. As reported in a large retrospective review of 2,022 patients by Myers and colleagues, certain life-threatening complications have been reported with the use of JTs, including torsion and bowel necrosis at an estimated rate of 0.4% of patients (83). Overall tube-related complications occurred in 1.5% of patients with the most common complications being either occlusion or dislodgement in 0.7% of patients. The intra-abdominal infection rate was reported to be 0.8%. Gerritsen et al. [2012] found JTs to have the lowest wound infection rate (6%) compared to the NJT group (16%) and PN group (30%) (P=0.02) (75). Interestingly, in the systematic review by Gerritsen and colleagues [2013], the JT group had the lowest mean overall morbidity rate at 43.8% (56).
Pancreatic fistula
PF is one of the most serious complications following pancreatic cancer surgery. The definition varies widely in the literature, although two of the most common definitions include >10 cc/day of amylase rich fluid after postoperative day 3 or continued drainage of amylase rich fluid after postoperative day 20 as defined by the international study group on pancreatic fistula (ISGPF) (84). Schmidt et al. evaluated various risk factors for the development of PF following PD in a series of 510 patients (85). A total of 46 PFs developed postoperatively. Interestingly, the use of mechanical bowel preparation was found to be protective against development of a PF (6% vs. 19%, P<0.02). On multivariate analysis, risk factors for PF formation included invaginated pancreatico-jejunostomies (OR =3.30, P=0.01) and closed suction drainage (OR =2.24, P=0.05). Factors protective against PF formation included pancreatitis (OR =0.22, P=0.05) and preoperative endoscopic biliary stenting (OR =0.34, P=0.05). As expected in this series, patients with PFs were more likely to develop septic complications, longer hospitalizations, and a higher incidence of reoperations.
Methods to treat PF from a nutritional standpoint have been previously discussed. Although both EN and PN have been used to assist in closure of PFs, EN is clearly superior with a shorter median time to closure than PN (70). The only predictors of closure were EN and initial fistula output ≤200 cc/day.
Future endeavors
The evolution of pancreatic surgery over the last three decades has led to significant improvements in morbidity and mortality. Improving patients’ perioperative nutritional status is a realistic target to further improve outcomes and quality of life. Many questions remain. For example, what is the best measure of malnutrition in patients with pancreatic cancer and what parameters should be used to signal the optimal time for surgery in the malnourished patient? What should be the duration of preoperative nutrition in the malnourished patient, and should it be per os or via a tube? Are NJT feeds in fact superior to other forms of postoperative enteric alimentation following pancreatic cancer surgery? Is there potentially a role in placing a gastric stimulator or performing a sleeve gastrectomy at the time of surgery in patients with either known gastroparesis or those at significantly increased risk of developing DGE? Does enzyme replacement play a role during the perioperative period? Do any interventions short of returning the patient to balanced nutrition result in decreased morbidity and mortality? These questions will help further our understanding of the impact of nutrition on this patient population; this requires a commitment from the field, as these questions are unlikely to be resolved by individual centers. Defining feed strategies and categorizing success and failure after pancreatic surgery should be considered by the International Study Group of Pancreatic Surgery.
Conclusions
Nutrition plays an integral role in pancreatic cancer surgery, not only preoperatively, but also in the postoperative period. A multidisciplinary approach to assess preoperative nutrition helps determine which patients may require additional support in the perioperative period. We believe oral feeding at will remains the best approach based on available randomized control trials and observational studies in pancreatic surgery, and literature from other surgical disciplines. This approach provides nourishment and hydration, though has not been clearly demonstrated to provide balanced nutrition. Enteral feeding tubes should be used in select cases. The choice of feeding tube should be the NJT if possible, as the major morbidity profile is the least. There does not appear to be benefits from routine use of NGTs for decompression. PN should be reserved for patients in whom it is not possible to obtain enteral access for feeding. Mitigating postoperative complications, including DGE and PF, remain of utmost importance to maximize outcomes in patients undergoing pancreatic surgery. Future endeavors should focus on better identifying those patients who might benefit from perioperative supplementation of nutrition, which specific enteral feeding route, and the timing of placement.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Whipple AO. The rationale of radical surgery for cancer of the pancreas and ampullary region. Ann Surg 1941;114:612-5. [PubMed]
- Cameron JL, Riall TS, Coleman J, et al. One thousand consecutive pancreaticoduodenectomies. Ann Surg 2006;244:10-5. [PubMed]
- Ansorge C, Lindström P, Strömmer L, et al. Assessing surgical quality: comparison of general and procedure-specific morbidity estimation models for the risk adjustment of pancreaticoduodenectomy outcomes. World J Surg 2014;38:2412-21. [PubMed]
- Afaneh C, O’Mahoney P, Giambrone G, et al. Mo1617 Population-Based Trends of Pancreaticoduodenectomy: Temporal and Age-Related Outcomes. Gastroenterology 2014;146:S-1067.
- House MG, Fong Y, Arnaoutakis DJ, et al. Preoperative predictors for complications after pancreaticoduodenectomy: impact of BMI and body fat distribution. J Gastrointest Surg 2008;12:270-8. [PubMed]
- Schmidt CM, Turrini O, Parikh P, et al. Effect of hospital volume, surgeon experience, and surgeon volume on patient outcomes after pancreaticoduodenectomy: A single-institution experience. Arch Surg 2010;145:634-40. [PubMed]
- Greenblatt DY, Kelly KJ, Rajamanickam V, et al. Preoperative factors predict perioperative morbidity and mortality after pancreaticoduodenectomy. Ann Surg Oncol 2011;18:2126-35. [PubMed]
- Muscari F, Suc B, Kirzin S, et al. Risk factors for mortality and intra-abdominal complications after pancreatoduodenectomy; multivariate analysis in 300 patients. Surgery 2006;139:591-8. [PubMed]
- Sierzega M, Niekowal B, Kulig J, et al. Nutritional status affects the rate of pancreatic fistula after distal pancreatectomy: a multivariate analyses of 132 patients. J Am Coll Surg 2007;205:52-9. [PubMed]
- Schnelldorfer T, Mauldin PD, Lewin DN, et al. Distal pancreatectomy for chronic pancreatitis: risk factors for postoperative pancreatic fistula. J Gastrointest Surg 2007;11:991-7. [PubMed]
- Mourão F, Amado D, Ravasco P, et al. Nutritional risk and status assessment in surgical patients: A challenge amidst plenty. Nutr Hosp 2004;19:83-8. [PubMed]
- Afaneh C, Rich B, Aull MJ, et al. Pancreas transplantation considering the spectrum of body mass indices. Clin Transplant 2011;25:E520-9. [PubMed]
- La Torre M, Ziparo V, Nigri G, et al. Malnutrition and Pancreatic Surgery: Prevalence and Outcomes. J Surg Oncol 2013;107:702-8. [PubMed]
- Ahmad SA, Edwards MJ, Sutton JM, et al. Factors influencing readmission after pancreaticoduodenectomy: a multi-institutional study of 1302 patients. Ann Surg 2012;256:529-37. [PubMed]
- Berry AJ. Pancreatic surgery: indications, complications, and implications for nutrition intervention. Nutr Clin Pract 2013;28:330-57. [PubMed]
- Porta M, Fabregat X, Malats N, et al. Exocrine pancreatic cancer: symptoms at presentation and their relation to tumour site and stage. Clin Transl Oncol 2005;7:189-97. [PubMed]
- Kanda M, Fujii T, Kodera Y, et al. Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg 2011;98:268-74. [PubMed]
- Karagianni VT, Papalois AE, Triantafillidis JK. Nutritional status and nutritional support before and after pancreatectomy for pancreatic cancer and chronic pancreatitis. Indian J Surg Oncol 2012;3:348-59. [PubMed]
- Studley HO. Percentage of weight loss: a basic indicator of surgical risk in patients with chronic peptic ulcer. 1936. Nutr Hosp 2001;16:141-3; discussion 140-1. [PubMed]
- White JV, Guenter P, Jensen G, et al. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J Acad Nutr Diet 2012;112:730-8. [PubMed]
- Detsky AS, McLaughlin JR, Baker JP, et al. What is subjective global assessment of nutritional status? JPEN J Parenter Enteral Nutr 1987;11:8-13. [PubMed]
- Bauer J, Capra S, Ferguson M. Use of the scored patient-generated subjective global assessment (PG-SGA) as a nutrition assessment tool in patients with cancer. Eur J Clin Nutr 2002;56:779-85. [PubMed]
- Velasco C, García E, Rodríguez V, et al. Comparison of four nutritional screening tools to detect nutritional risk in hospitalized patients: a multicentre study. Eur J Clin Nutr 2011;65:269-74. [PubMed]
- Vigano AL, Di Tomasso J, Kilgour RD, et al. The abridged patient-generated subjective global assessment is a useful tool for early detection and characterization of cancer cachexia. J Acad Nutr Diet 2014;114:1088-98. [PubMed]
- Loh KW, Vriens MR, Gerritsen A, et al. Unintentional weight loss is the most important indicator of malnutrition among surgical cancer patients. Neth J Med 2012;70:365-9. [PubMed]
- Almeida AI, Correia M, Camila M, et al. Nutritional risk screening in surgery: Valid, feasible, easy! Clin Nutr 2012;31:206-11. [PubMed]
- Faramarzi E, Mahdavi R, Mohammad-Zadeh M, et al. Validation of nutritional risk index method against patient-generated subjective global assessment in screening malnutrition in colorectal cancer patients. Chin J Cancer Res 2013;25:544-8. [PubMed]
- Shinkawa H, Takemura S, Uenishi T, et al. Nutritional risk index as an independent predictive factor for the development of surgical site infection after pancreaticoduodenectomy. Surg Today 2013;43:276-83. [PubMed]
- World Health Organization. eds. Obesity: preventing and managing the global epidemic. Geneva: World Health Organization, 2000.
- Forse RA, Shizgal HM. Serum albumin and nutritional status. JPEN J Parenter Enteral Nutr 1980;4:450-4. [PubMed]
- Hennessey DB, Burke JP, Ni-Dhonochu T, et al. Preoperative hypoalbuminemia is an independent risk factor for the development of surgical site infection following gastrointestinal surgery: a multi-institutional study. Ann Surg 2010;252:325-9. [PubMed]
- Takasu C, Shimada M, Kurita N, et al. Impact of C-reactive protein on prognosis of patients with colorectal carcinoma. Hepatogastroenterology 2013;60:507-11. [PubMed]
- Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol 2010;6:149-63. [PubMed]
- Jamieson NB, Glen P, McMillan DC, et al. Systemic inflammatory response predicts outcome in patients undergoing resection for ductal adenocarcinoma head of pancreas. Br J Cancer 2005;92:21-3. [PubMed]
- McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care 2009;12:223-6. [PubMed]
- Glen P, Jamieson NB, McMillan DC, et al. Evaluation of an inflammation-based prognostic score in patients with inoperable pancreatic cancer. Pancreatology 2006;6:450-3. [PubMed]
- Lassen K, Coolsen MM, Slim K, et al. ERAS® Society; European Society for Clinical Nutrition and Metabolism; International Association for Surgical Metabolism and Nutrition. Guidelines for perioperative care for pancreaticoduodenectomy: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Clin Nutr 2012;31:817-30. [PubMed]
- Carli F, Charlebois P, Baldini G, et al. An integrated multidisciplinary approach to implementation of a fast-track program for laparoscopic colorectal surgery. Can J Anaesth 2009;56:837-42. [PubMed]
- Mechanick JI, Youdim A, Jones DB, et al. AACE/TOS/ASMBS Clinical Practice Guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient – 2013 update: cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and America Society for Metabolic & Bariatric Surgery. Endocr Pract 2013;19:S1-S27.
- Correia MI, Caiaffa WT, da Silva AL, et al. Risk factors for malnutrition in patients undergoing gastroenterological and hernia surgery: an analysis of 374 patients. Nutr Hosp 2001;16:59-64. [PubMed]
- Bozzetti F, Gianotti L, Braga M, et al. Postoperative complications in gastrointestinal cancer patients: the joint role of the nutritional status and the nutritional support. Clin Nutr 2007;26:698-709. [PubMed]
- Zhou W, Xu X, Yan J, et al. Nutritional risk is still a clinical predictor of postoperative outcomes in laparoscopic abdominal surgery. Surg Endosc 2013;27:2569-74. [PubMed]
- Wu GH, Liu ZH, Wu ZH, et al. Perioperative artificial nutrition in malnourished gastrointestinal cancer patients. World J Gastroenterol 2006;12:2441-4. [PubMed]
- Watters JM, Kirkpatrick SM, Norris SB, et al. Immediate postoperative enteral feeding results in impaired respiratory mechanics and decreased mobility. Ann Surg 1997;226:369-77. [PubMed]
- Weimann A, Braga M, Harsanyi L, et al. ESPEN Guidelines on Enteral Nutrition: Surgery including organ transplantation. Clin Nutr 2006;25:224-44. [PubMed]
- Bozzetti F. Perioperative nutritional support in the ERAS approach. Clin Nutr 2013;32:872-3. [PubMed]
- Wichmann MW, Roth M, Jauch KW, et al. A prospective clinical feasibility study for multimodal “fast track” rehabilitation in elective pancreatic cancer surgery. Rozhl Chir 2006;85:169-75. [PubMed]
- Kennedy EP, Rosato EL, Sauter PK, et al. Initiation of a critical pathway for pancreaticoduodenectomy at an academic institution: the first step in multidisciplinary team building. J Am Coll Surg 2007;204:917-23. [PubMed]
- Berberat PO, Ingold H, Gulbinas A, et al. Fast track different-implications in pancreatic surgery. J Gastrointest Surg 2007;11:880-7. [PubMed]
- Balzano G, Zerbi A, Braga M, et al. Fast- track recovery programme after pancreaticoduodenectomy reduces delayed gastric emptying. Br J Surg 2008;95:1387-93. [PubMed]
- Montiel Casado MC, Pardo SF, Rotellar SF, et al. Experience of a cephalic pancreatoduodenectomy fast-track program. Cir Esp 2010;87:378-84. [PubMed]
- di Sebastiano P, Festa L, De Bonis A, et al. A modified fast-track program for pancreatic surgery: a prospective single-center experience. Langenbecks Arch Surg 2011;396:345-51. [PubMed]
- Robertson N, Gallacher PJ, Peel N, et al. Implementation of an enhanced recovery programme following pancreaticoduodenectomy. HPB (Oxford) 2012;14:700-8. [PubMed]
- Lassen K, Ljungqvist O, Dejong CH, et al. Pancreaticoduodenectomy: ERAS recommendations. Clin Nutr 2013;32:870-1. [PubMed]
- Braga M, Pecorelli N, Ariotti R, et al. Enhanced Recovery After Surgery Pathway in Patients Undergoing Pancreaticoduodenectomy. World J Surg 2014;38:2960-6. [PubMed]
- Gerritsen A, Besselink MGH, Gouma DJ, et al. Systematic review of five feeding routes after pancreatoduodenectomy. Br J Surg 2013;100:589-98; discussion 599. [PubMed]
- Brennan MF, Pisters PW, Posner M, et al. A prospective randomized trial of total parenteral nutrition after major pancreatic resection for malignancy. Ann Surg 1994;220:436-41; discussion 441-4. [PubMed]
- Martignoni ME, Friess H, Sell F, et al. Enteral nutrition prolongs delayed gastric emptying in patients after Whipple resection. Am J Surg 2000;180:18-23. [PubMed]
- Mack LA, Kaklamanos IG, Livingstone AS, et al. Gastric decompression and enteral feeding through a double-lumen gastrojejunostomy tube improves outcomes after pancreaticoduodenectomy. Ann Surg 2004;240:845-51. [PubMed]
- Lewis SJ, Egger M, Sylvester PA, et al. Early enteral feeding versus “nil by mouth” after gastrointestinal surgery: systematic review and metaanalysis of controlled trials. BMJ 2001;323:773-6. [PubMed]
- Ronellenfitsch U, Schwarzbach M, Kring A, et al. The effect of clinical pathways for bariatric surgery on perioperative quality of care. Obes Surg 2012;22:732-9. [PubMed]
- Lassen K, Kjaeve J, Fetveit T, et al. Allowing normal food at will after major upper gastrointestinal surgery does not increase morbidity a randomized multicenter trial. Ann Surg 2008;247:721-9. [PubMed]
- ASPEN Board of Directors and The Clinical Guidelines Task Force. Guidelines for the use of parenteral and enteral nutrition in adult and pediatric patients. JPEN J Parenter Enteral Nutr 2002;26:1SA-138SA.
- Braga M, Ljungqvist O, Soeters P, et al. ESPEN Guidelines for parenteral nutrition: surgery. Clinical Nutrition 2009;28:378-86. [PubMed]
- Casaer MP, Mesotten D, Hermans G, et al. Early versus late parenteral nutrition in critically ill adults. N Engl J Med 2011;365:506-17. [PubMed]
- Kritchevsky SB, Braun BI, Kusek L, et al. The impact of hospital practice on central venous catheter associated bloodstream infection rates at the patient and unit level: a multicenter study. Evaluation of Processes and Indicators in Infection Control (EPIC) Study Group. Am J Med Qual 2008;23:24. [PubMed]
- Amrutkar PP, Rege MD, Chen H, et al. Comparison of risk factors for candidemia versus bacteremia in hospitalized patients. Infection 2006;34:322. [PubMed]
- Gianotti L, Braga M, Gentilini O, et al. Artificial nutrition after pancreaticoduodenectomy. Pancreas 2000;21:344-51. [PubMed]
- Klek S, Sierzega M, Szybinski P, et al. Perioperative nutrition in malnourished surgical cancer patients- a prospective randomized, controlled, clinical trial. Clin Nutr 2011;30:708-13. [PubMed]
- Klek S, Sierzega M, Turczynowski L, et al. Enteral and parenteral nutrition in the conservative treatment of pancreatic fistula: a randomized clinical trial. Gastroenterology 2011;141:157-63. [PubMed]
- Moore FA, Feliciano DV, Andrassy RJ, et al. Early enteral feeding, compared with parenteral, reduces postoperative septic complications. The results of a meta-analysis. Ann Surg 1992;216:172-83. [PubMed]
- Osland E, Yunus RM, Khan S, et al. Early versus traditional postoperative feeding in patients undergoing resectional gastrointestinal surgery: a meta-analysis. JPEN J Parenter Enteral Nutr 2011;35:473-87. [PubMed]
- Braga M, Gianotti L, Gentilini O, et al. Early postoperative enteral nutrition improves gut oxygenation and reduces costs compared with total parenteral nutrition. Crit Care Med 2001;29:242-8. [PubMed]
- Zhu X, Wu Y, Qiu Y, et al. Comparative Analysis of the Efficacy and Complications of Nasojejunal and Jejunostomy on Patients Undergoing Pancreaticoduodenectomy. JPEN J Parenter Enteral Nutr 2014;38:996-1002. [PubMed]
- Gerritsen A, Besselink MG, Cieslak KP, et al. Efficacy and complications of nasojejunal, jejunostomy and parenteral feeding after pancreaticoduodenectomy. J Gastrointest Surg 2012;16:1144-51. [PubMed]
- Abu-Hilal M, Hemandas AK, McPhail M, et al. A comparative analysis of safety and efficacy of different methods of tube placement for enteral feeding following major pancreatic resection. A non-randomized study. JOP 2010;11:8-13. [PubMed]
- Scaife CL, Hewitt KC, Mone MC, et al. Comparison of intraoperative versus delayed enteral feeding tube placement in patients undergoing a Whipple procedure. HPB (Oxford) 2014;16:62-9. [PubMed]
- Nelson R, Tse B, Edwards S. Systematic review of prophylactic nasogastric decompression after abdominal operations. Br J Surg 2005;92:673-80. [PubMed]
- Fisher WE, Hodges SE, Guillermina C, et al. Routine nasogastric suction may be unnecessary after a pancreatic resection. HPB 2011;13:792-6. [PubMed]
- Kunstman JW, Klemen ND, Fonseca AL, et al. Nasogastric drainage may be unnecessary after pancreaticoduodenectomy: a comparison of routine vs selective decompression. J Am Coll Surg 2013;217:481-8. [PubMed]
- Malleo G, Crippa S, Butturini G, et al. Delayed gastric emptying after pylorus-preserving pancreaticoduodenectomy: validation of International Study Group of Pancreatic Surgery classification and analysis of risk factors. HPB (Oxford) 2010;12:610-8. [PubMed]
- Welsch T, Borm M, Degrate L, et al. Evaluation of the International Study Group of Pancreatic Surgery definition of delayed gastric emptying after pancreatoduodenectomy in a high-volume centre. Br J Surg 2010;97:1043-50. [PubMed]
- Myers JG, Page CP, Stewart RM, et al. Complications of needle catheter jejunostomy in 2,022 consecutive applications. Am J Surg 1995;170:547-50; discussion 550-1. [PubMed]
- Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group definition. Surgery 2005;138:8-13. [PubMed]
- Schmidt CM, Choi J, Powell ES, et al. Pancreatic fistula following pancreaticoduodenectomy: clinical predictors and patient outcomes. HPB Surg 2009;2009:404520.



