“Vanishing liver metastases”—A real challenge for liver surgeons
Introduction
Colorectal cancer (CRC) is one of the most common cancers in the world. Advances in treatment have led to a decrease in the death rate for CRC over past two decades (1). Despite these advances, approximately half of all patients diagnosed with CRC will develop liver metastasis (LM) during the course of their disease (2-5). When left untreated, colorectal LM is rapidly and uniformly fatal with a median survival measured in months (6,7). Surgical resection provides the best opportunity for long-term survival and even the chance for cure, and so it is the current paradigm of treatment (8-17). Unfortunately, only 10-25% of patients with LM are candidates for surgical resection at the time of presentation (18-20). In patients with unresectable metastases, chemotherapy is the treatment of choice, either as a palliative treatment or in attempt to convert them into surgical candidates (21-24). Chemotherapy can also be administrated as a neoadjuvant strategy for selected cases of colorectal LM (23-25). Thus, an increasing number of patients receive chemotherapy prior to liver resection (26,27). The introduction of new, more effective systemic cytotoxic and biologic agents have been an important advance in the management of CLM. Tumor response has significantly improved with modern combination regimens, with up to 50% response rates for unresectable LM and 20% proceeding to liver resection with curative intent (28). Along with an improvement in chemotherapy treatment, there has been an increasing evidence of “disappearing” liver metastases (DLM) (27-31). DLM defined as a disappearance of liver metastases on cross-sectional imaging after administration of preoperative chemotherapy, which means a complete radiological response. That phenomenon occurs in 5-38% of patients who undergo preoperative systemic therapy (27,29-32). The logic basis behind the decision-making algorithm for DLM built on understanding of correlation between the complete radiological response and complete pathological response or durable complete clinical response. The complete pathological response defined as an absence of residual tumor in the resection specimen. The durable complete clinical response means no recurrence during a satisfactory period of time, when the site of disappearing lesion in not resected (left in situ). Both are desirable outcomes promising a chance for cure.
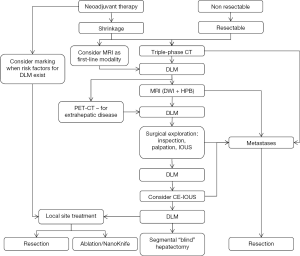
In this review we propose a decision-making algorithm for management of DLM which discuss step-by-step how to improve a clinical approach to DLM, emphasizing upfront improvements in imaging, intraoperative detection and surgical techniques.
DLM prevention—Overtreatment is not advisable
The reported risk factors for the occurrence of DLM are: small size of LM (<2 cm), initial number of metastasis (over 3) and a prolonged preoperative chemotherapy (26,30). Therefore, for those patients selected to receive neoadjuvant treatment for a resectable disease, preoperative chemotherapy should be given for a fixed short duration. There is no strict evidence for number of cycles to treat. Van Vledder and colleagues showed that patients with DLM received 7.7 cycles of chemotherapy versus 5.5 cycles in patients without DLM (26). In addition, an 18% increase in chance of DLM with each additional cycle of treatment was noted. The majority of DLMs arose 3-6 months following the start of chemotherapy (25). Based on that evidence, some writers proposed the numbers of 4-6 cycles (32,33). After that initial course the clinician should reevaluate the patient in order to avoid disappearing and to promptly resect it. It is important to remember, that patients that receive chemotherapy for resectable disease do not need to demonstrate objective response, although radiological response is a good prognostic factor. As far as conversion treatment for unresectable disease concerned, it should be continued until the patient has a resectable disease, not until maximum response (28,33).
In fact, prolonged chemotherapy can cause liver toxicity, and thus to disturb the management of LM by two mechanisms. First, it leads to decreased ability of preoperative imaging to detect LM, by increased fatty content (26,33,34). Second, it makes the surgery more difficult technically, causing an obvious increase in intra and postoperative morbidity (23).
Preoperative imaging—Are the metastases missing indeed?
The rate of complete radiological response varies in different series as much as 4% and 38% (25,26,28,33-37). It can be explained by differences in chemotherapy regimens and by the quality and competence of preoperative imaging. Numbers of modalities are in use to image patients with LM.
Computed tomography (CT)
Since its introduction into clinical practice in the 1970s, the quality and accuracy of CT in detecting LM has continued to improve, with a sensitivity ranging currently between 63% and 90%, and specificity between 85% and 90%, approaching 100% in some series (26,33,34,38,39).
Preoperative chemotherapy can induce parenchymal changes to the liver, defined as steatosis or steatohepatitis (33,40). In that setting the background liver appears darker, allowing less contrast between the parenchyma and the hypovascular metastases, hindering their detection (34,41,42).
Several risk factors have been reported causing an inadequate staging of LM by CT, such as steatosis > 30%, more than 3 LM and lesions smaller than 1 cm (32-34).
Based on this evidence, we assume that all missing metastases on triple-phase CT should be confirmed by another imaging modality.
PET-FDG and PET-CT
That modality shows high sensitivity, up to 97%, for detecting LM in some series (43,44). Other publications reported wider range of sensitivities—51-90% (40,45-48). This data reflects several factors, which reduce the sensitivity of FDG uptake, such as small lesions (especially less than 1 cm) and impaired glucose uptake in tumor cells due to chemotherapy (49,50). Nevertheless, some series emphasized an important role of PET-FDG, changing the treatment plan in up to 30-40%, either by finding an extrahepatic disease or correctly predicting a complete pathological response (51,52). In a prospective study of 104 patients with CRC, PET-CT revealed unsuspected disease in 19%, changed stage in 13.5% and resulted in modified surgery in 11.5% (53). As the likelihood of extrahepatic disease increases along with the degree of liver involvement, PET-CT should be considered as a routine examination in staging patients prior to surgical resection (34). This is important when considering extensive surgery to avoid the morbidity of futile laparotomies.
In summary, remaining an important tool in primary staging, PET scan is not a good test for looking at viable cancer within the liver after chemotherapy (54).
MRI
MRI appears to be the best hepatic imaging modality, especially in the setting of chemotherapy-induced steatosis and for small lesions (28,55). Compared with CT, it has better sensitivity and specificity (34). Recent advances in MRI techniques, such as diffusion-weighted imaging (DWI) and hepatobiliary contrast agents even strengthen that superiority. DWI is a measure of the ability of water molecules to diffuse freely between tissues and hence directly correlates with underlying cellular density. Metastases tend to restrict diffusion and the addition of DWI to the typical liver MRI protocol improves sensitivity and specificity for lesion detection and characterization (56-60). In addition to DWI, hepatobiliary phase MRI using liver-specific contrasts has demonstrated improved sensitivity to metastasis detection over routine MRI (61-64). Examples of such contrast agents are Gadoxetic acid and super paramagnetic iron oxide (SPIO). These agents help to improve the contrast between hepatocytes and tumor cells during the late hepatobiliary phase, in which peak parenchymal enhancement happens.
In summary, MRI is an optimal modality to image LM missing on CT scan. Moreover, in recent study an inability to observe a DLM on MRI was associated with an increased chance for complete pathological or durable clinical response (30).
Following adequate imaging—Should we always operate?
Since no preoperative imaging modality, including MRI, has a sensitivity of 100%, there is a subset of DLM that will be found only at the time of surgical exploration. In other words, if we do not proceed to surgical exploration in setting of DLM even after performing comprehensive imaging, we may leave the tumor behind. So one should always consider surgical exploration when feasible, especially in presence of DLM risk factors, mentioned previously, such as small and multiple lesions, prolonged chemotherapy and significant chemotherapy induced liver damage. The literature hasn’t faced the difference between per patient versus per metastases approach to exploration. Obviously, a patient with multiple metastases, which only part of them disappeared on imaging, will undergo exploration, demanding resection of remaining lesions in any case. It is less clear how safe is a possibility to avoid surgery in rare patient with completely “clean” post-chemotherapy liver. Such specific cases should be discussed in a multidisciplinary team, taking in consideration favorable prognosis in good treatment responders. That fact promotes an aggressive approach with meticulous intraoperative assessment.
Intraoperative assessment—Could we do better?
The role of exploratory laparoscopy as a first step in operative approach to DLM is still being controversial. The main importance of laparoscopy in such cases is probably to rule out a disseminated peritoneal disease. The ability of laparoscopy to identify small lesions missing on preoperative imaging is significantly limited.
Using formal laparotomy, all patients with DLM should undergo a full liver mobilization, visual inspection, palpation and finally intraoperative ultrasonography (IOUS). Systematic examination by IOUS can lead to an increase in the detection of DLM. In the published experience, a macroscopic residual disease was observed in as much as 27-45% of the patients with DLM by combination of palpation and IOUS (25,26,36,37). As mentioned previously, that frequency was lowered by the use of preoperative MRI (26,28,36).
Contrast-enhanced intraoperative ultrasound (CE-IOUS) is a novel technique that was proposed in 2004 for both CLM and hepatocellular carcinoma detection. The preliminary results were inconclusive for CLM (64-66). Further investigations showed that it is capable of detecting a larger proportion of CLM, in comparison with other imaging modalities including IOUS (53,67,68). Arita et al. assessed a usefulness of CE-IOUS in identifying DLM (69). Out of 32 DLM, 4 were identified by IOUS, all confirmed as tumor by pathology. Out of remaining 28, 12 we found by CE-IOUS, all were resected and a vast majority (11 of 12) consisted of malignancy. The authors concluded that CE-IOUS might be necessary for identifying DLM.
Possible factors influencing the surgeon ability to discover DLM include the degree of hepatic steatosis, the depth of DLM, the location relative to anatomical landmarks and surgeon skill with IOUS (28,66,70).
How to treat missing LM during surgery
When a surgeon cannot identify DLM during the operation, he has two options to manage that situation. First is to treat surgically the site of anatomical location of the metastases, and check for complete pathological response in pathology regimen. Second, he can leave it in situ. In that scenario the outcome will be assessed by the follow-up imaging, looking for recurrence al the site of DLM. The duration of the follow-up to define a complete clinical response is not well defined. According to the fact that the median time of recurrence is 6-8 months, it is makes sense to define a durable clinical response as no recurrence at cross-sectional imaging at 1 year (26,28,30).
The literature is not is not convincing when facing the dilemma of resecting the site of metastasis versus leaving it in situ. Several predicting factors for a good correlation between a complete radiological and complete pathological response were described. Most significant of them were initial higher number of LM, more metastases with partial response, young patients (<60 years), low initial CEA level (<30) which normalizes during chemotherapy, small lesions (<3 cm) and an absence of lesion on preoperative MRI (26,37). Another independent predicting factor was a use of hepatic artery infusion (HAI) therapy (28,36,37).
Proponents of aggressive resection present high rates of recurrence while left in situ (above 70%) and low rates of complete pathological response when resected (20%) (27,28). van Vledder et al. showed a significant advantage in 3-year intrahepatic recurrence-free survival rates for resection versus follow-up group (26). On the other hand, there was no difference in overall survival (26,27). The possible explanation is the fact that about a half of the patients experience recurrence in any other location, different from the DLM sites or even extrahepatic (33). The aggressive biologic nature of disease in those patients may neutralize the local control of disease by DLM sites resection, thus moderating overall survival benefit (25,26,33).
From the practical point of view, the decision should be made based on aggressiveness of the disease, the patient condition and operative risk, an ability to treat all sites surgically and predictive factors for true complete pathological response as described above.
Advances in surgeon arsenal—From “blind” hepatectomy to NanoKnife
When the lesion cannot be identified, incorporation of the original site to hepatectomy or even performing segmental hepatectomy for a DLM site alone should be considered (26). The clear disadvantage of such “blind” hepatectomy technique is an inadequate residual liver volume and increased surgical risk. In fact, performing a major hepatectomy to resect the site of the DLM may not decrease the recurrence rate (27). On the contrary, the prognosis could be worsened by reducing the possibility of second hepatectomy. Along with the general trend of liver sparing in hepatobiliary surgery, in the field of DLM technological improvements allow more precise intervention. The key point is an exact site location. One option is to mark the LM with coils using percutaneous interventional radiology techniques (71). Although discussed in the chapter of operative treatment, its real place in decision-making algorithm is before starting chemotherapy. One can consider that tool, when dealing with an aggressive disease, which requires prolonged therapy, or when mentioned risk factors for DLM exist. Additional aids to assist in surgical planning are new software and applications that alleviate determining surgical planes, evaluating FLR and depicting anatomy (34).
Radiofrequency ablation (RFA) is a gaining momentum alternative for liver lesion resection (72). The idea of ablating a previously marked site of DLM is promising in avoiding massive resections. It is timely influencing the debate about the necessity of DLM site resection. The problem in analyzing that modality is to compare it to surgical resections. Unlike in surgical resection, an evidence for complete response rates can be collected by looking for recurrence in follow-up imaging.
In spite of its widespread use and noted efficacy, RFA has some limitations. Its dependence on heating of the tissue to denature proteins means adjacent thermosensitive structures such as colon, stomach, bile ducts, gallbladder, and hepatic capsule can be damaged resulting in complications, and large vessels within or close to the treatment zone may cause thermal sinks (“heat-sink” effect) that will prevent complete treatment of the target lesion (72,73). Although there are new thermal technologies such as microwave ablation, which may potentially generate a larger ablation zone in a shorter time, they still have the limitations associated with thermal technologies. These limitations have generated interest in other methods of ablation and have forced an integration of irreversible electroporation (IRE) method into treatment options list of hepatic tumors.
IRE, commercially available as NanoKnife, is a new ablative technology that uses high-voltage, low-energy DC current to create nanopores in the cell membrane, disrupting the homeostasis mechanism and inducing cell death by initiating apoptosis (74). Its major advantage is the lack of heat-sink effect and the ability to treat zones near vessels, bile ducts, and critical structures. IRE comes with its own share of limitations. Human experiences are still limited, whereas thermal ablative techniques such as RFA have been time-tested for nearly three decades. The procedure has a learning curve because multiple needle placements are required within a prescribed distance, which can be challenging, and parallel placement of the probes may be hindered by issues, such as intervening ribs. In addition, this is a very expensive technology. We doubt a routine use of it when dealing with the lesion that is not even visible and the need for resection is controversial.
Computer assisted liver surgery can be an elegant way to locate and ablate the site of DLM. Indeed, the integration of the prechemotherapy imaging to the US imaging along with the navigation system can allow the surgeon to locate and ablate precisely the metastatic site (75).
Summary
Our review suggest an algorithm for clinical approach to DLM (Figure 1). The most crucial steps are a comprehensive preoperative imaging, including MRI, careful surgical exploration, using IOUS and possibly CE-IOUS, and to be assisted by variety of operative techniques, such as local ablation of previously marked sites. The algorithm might serve as a helpful tool, but it definitely does not replace a multidisciplinary team, which should carry out the treatment of such a complicated patients. As the technology is improving fast, we look forward for the future improvements. The desirable navigation system may give an answer for difficulties to locate previous sites of DLM.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- American Cancer Society. Detailed Guide: Colon and Rectum Cancer page. Available online: http://www.cancer.org, accessed 4/15/2010.
- Van Cutsem E, Nordlinger B, Adam R, et al. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur J Cancer 2006;42:2212-21. [PubMed]
- Jemal A, Murray T, Ward E, et al. Cancer statistics, 2005. CA Cancer J Clin 2005;55:10-30. [PubMed]
- Stangl R, Altendorf-Hofmann A, Charnley RM, et al. Factors influencing the natural history of colorectal liver metastases. Lancet 1994;343:1405-10. [PubMed]
- Leonard GD, Brenner B, Kemeny NE. Neoadjuvant chemotherapy before liver resection for patients with unresectable liver metastases from colorectal carcinoma. J Clin Oncol 2005;23:2038-48. [PubMed]
- Bengmark S, Hafström L. The natural history of primary and secondary malignant tumors of the liver. I. The prognosis for patients with hepatic metastases from colonic and rectal carcinoma by laparotomy. Cancer 1969;23:198-202. [PubMed]
- Bengtsson G, Carlsson G, Hafström L, et al. Natural history of patients with untreated liver metastases from colorectal cancer. Am J Surg 1981;141:586-9. [PubMed]
- Alberts SR, Poston GJ. Treatment advances in liver-limited metastatic colorectal cancer. Clin Colorectal Cancer 2011;10:258-65. [PubMed]
- Berri RN, Abdalla EK. Curable metastatic colorectal cancer: recommended paradigms. Curr Oncol Rep 2009;11:200-8. [PubMed]
- Kopetz S, Vauthey JN. Perioperative chemotherapy for resectable hepatic metastases. Lancet 2008;371:963-5. [PubMed]
- Scheele J, Stang R, Altendorf-Hofmann A, et al. Resection of colorectal liver metastases. World J Surg 1995;19:59-71. [PubMed]
- Fortner JG, Silva JS, Golbey RB, et al. Multivariate analysis of a personal series of 247 consecutive patients with liver metastases from colorectal cancer. I. Treatment by hepatic resection. Ann Surg 1984;199:306-16. [PubMed]
- Fong Y, Cohen AM, Fortner JG, et al. Liver resection for colorectal metastases. J Clin Oncol 1997;15:938-46. [PubMed]
- Hughes KS, Simon R, Songhorabodi S, et al. Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of patterns of recurrence. Surgery 1986;100:278-84. [PubMed]
- Nordlinger B, Guiguet M, Vaillant JC, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Française de Chirurgie. Cancer 1996;77:1254-62. [PubMed]
- Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999;230:309-18; discussion 318-21. [PubMed]
- Figueras J, Valls C, Rafecas A, et al. Resection rate and effect of postoperative chemotherapy on survival after surgery for colorectal liver metastases. Br J Surg 2001;88:980-5. [PubMed]
- Scheele J. Hepatectomy for liver metastases. Br J Surg 1993;80:274-6. [PubMed]
- Petrelli NJ, Abbruzzese J, Mansfield P, et al. Hepatic resection: the last surgical frontier for colorectal cancer. J Clin Oncol 2005;23:4475-7. [PubMed]
- Adam R. Chemotherapy and surgery: new perspectives on the treatment of unresectable liver metastases. Ann Oncol 2003;14 Suppl 2:ii13-6. [PubMed]
- Meric F, Patt YZ, Curley SA, et al. Surgery after downstaging of unresectable hepatic tumors with intra-arterial chemotherapy. Ann Surg Oncol 2000;7:490-5. [PubMed]
- Adam R, Delvart V, Pascal G, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg 2004;240:644-57; discussion 657-8. [PubMed]
- Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet 2008;371:1007-16. [PubMed]
- Adam R, Wicherts DA, de Haas RJ, et al. Patients with initially unresectable colorectal liver metastases: is there a possibility of cure? J Clin Oncol 2009;27:1829-35. [PubMed]
- Allen PJ, Kemeny N, Jarnagin W, et al. Importance of response to neoadjuvant chemotherapy in patients undergoing resection of synchronous colorectal liver metastases. J Gastrointest Surg 2003;7:109-15; discussion 116-7. [PubMed]
- van Vledder MG, de Jong MC, Pawlik TM, et al. Disappearing colorectal liver metastases after chemotherapy: should we be concerned? J Gastrointest Surg 2010;14:1691-700. [PubMed]
- Benoist S, Brouquet A, Penna C, et al. Complete response of colorectal liver metastases after chemotherapy: does it mean cure? J Clin Oncol 2006;24:3939-45. [PubMed]
- Bischof DA, Clary BM, Maithel SK, et al. Surgical management of disappearing colorectal liver metastases. Br J Surg 2013;100:1414-20. [PubMed]
- Elias D, Goere D, Boige V, et al. Outcome of posthepatectomy-missing colorectal liver metastases after complete response to chemotherapy: impact of adjuvant intra-arterial hepatic oxaliplatin. Ann Surg Oncol 2007;14:3188-94. [PubMed]
- Auer RC, White RR, Kemeny NE, et al. Predictors of a true complete response among disappearing liver metastases from colorectal cancer after chemotherapy. Cancer 2010;116:1502-9. [PubMed]
- Lubezky N, Geva R, Shmueli E, et al. Is there a survival benefit to neoadjuvant versus adjuvant chemotherapy, combined with surgery for resectable colorectal liver metastases? World J Surg 2009;33:1028-34. [PubMed]
- Thomay AA, Charpentier KP. Optimizing resection for “responding” hepatic metastases after neoadjuvant chemotherapy. J Surg Oncol 2010;102:1002-8. [PubMed]
- Gaujoux S, Goéré D, Dumont F, et al. Complete radiological response of colorectal liver metastases after chemotherapy: what can we expect? Dig Surg 2011;28:114-20. [PubMed]
- Fowler KJ, Linehan DC, Menias CO. Colorectal liver metastases: state of the art imaging. Ann Surg Oncol 2013;20:1185-93. [PubMed]
- Chun YS, Vauthey JN, Ribero D, et al. Systemic chemotherapy and two-stage hepatectomy for extensive bilateral colorectal liver metastases: perioperative safety and survival. J Gastrointest Surg 2007;11:1498-504; discussion 1504-5. [PubMed]
- Elias D, Youssef O, Sideris L, et al. Evolution of missing colorectal liver metastases following inductive chemotherapy and hepatectomy. J Surg Oncol 2004;86:4-9. [PubMed]
- Tanaka K, Takakura H, Takeda K, et al. Importance of complete pathologic response to prehepatectomy chemotherapy in treating colorectal cancer metastases. Ann Surg 2009;250:935-42. [PubMed]
- Kamel IR, Choti MA, Horton KM, et al. Surgically staged focal liver lesions: accuracy and reproducibility of dual-phase helical CT for detection and characterization. Radiology 2003;227:752-7. [PubMed]
- Kim YK, Park G, Kim CS, et al. Diagnostic efficacy of gadoxetic acid-enhanced MRI for the detection and characterisation of liver metastases: comparison with multidetector-row CT. Br J Radiol 2012;85:539-47. [PubMed]
- Zorzi D, Laurent A, Pawlik TM, et al. Chemotherapy-associated hepatotoxicity and surgery for colorectal liver metastases. Br J Surg 2007;94:274-86. [PubMed]
- Oliva MR, Mortele KJ, Segatto E, et al. Computed tomography features of nonalcoholic steatohepatitis with histopathologic correlation. J Comput Assist Tomogr 2006;30:37-43. [PubMed]
- Albrecht T, Blomley MJ, Burns PN, et al. Improved detection of hepatic metastases with pulse-inversion US during the liver-specific phase of SHU 508A: multicenter study. Radiology 2003;227:361-70. [PubMed]
- Bipat S, van Leeuwen MS, Comans EF, et al. Colorectal liver metastases: CT, MR imaging, and PET for diagnosis--meta-analysis. Radiology 2005;237:123-31. [PubMed]
- Kinkel K, Lu Y, Both M, et al. Detection of hepatic metastases from cancers of the gastrointestinal tract by using noninvasive imaging methods (US, CT, MR imaging, PET): a meta-analysis. Radiology 2002;224:748-56. [PubMed]
- Niekel MC, Bipat S, Stoker J. Diagnostic imaging of colorectal liver metastases with CT, MR imaging, FDG PET, and/or FDG PET/CT: a meta-analysis of prospective studies including patients who have not previously undergone treatment. Radiology 2010;257:674-84. [PubMed]
- van Kessel CS, Buckens CF, van den Bosch MA, et al. Preoperative imaging of colorectal liver metastases after neoadjuvant chemotherapy: a meta-analysis. Ann Surg Oncol 2012;19:2805-13. [PubMed]
- Muhi A, Ichikawa T, Motosugi U, et al. Diagnosis of colorectal hepatic metastases: comparison of contrast-enhanced CT, contrast-enhanced US, superparamagnetic iron oxide-enhanced MRI, and gadoxetic acid-enhanced MRI. J Magn Reson Imaging 2011;34:326-35. [PubMed]
- Kim YK, Park G, Kim CS, et al. Diagnostic efficacy of gadoxetic acid-enhanced MRI for the detection and characterisation of liver metastases: comparison with multidetector-row CT. Br J Radiol 2012;85:539-47. [PubMed]
- Fong Y, Saldinger PF, Akhurst T, et al. Utility of 18F-FDG positron emission tomography scanning on selection of patients for resection of hepatic colorectal metastases. Am J Surg 1999;178:282-7. [PubMed]
- Tan MC, Linehan DC, Hawkins WG, et al. Chemotherapy-induced normalization of FDG uptake by colorectal liver metastases does not usually indicate complete pathologic response. J Gastrointest Surg 2007;11:1112-9. [PubMed]
- Huebner RH, Park KC, Shepherd JE, et al. A meta-analysis of the literature for whole-body FDG PET detection of recurrent colorectal cancer. J Nucl Med 2000;41:1177-89. [PubMed]
- Ruers TJ, Wiering B, van der Sijp JR, et al. Improved selection of patients for hepatic surgery of colorectal liver metastases with (18)F-FDG PET: a randomized study. J Nucl Med 2009;50:1036-41. [PubMed]
- Llamas-Elvira JM, Rodríguez-Fernández A, Gutiérrez-Sáinz J, et al. Fluorine-18 fluorodeoxyglucose PET in the preoperative staging of colorectal cancer. Eur J Nucl Med Mol Imaging 2007;34:859-67. [PubMed]
- Akhurst T, Kates TJ, Mazumdar M, et al. Recent chemotherapy reduces the sensitivity of [18F]fluorodeoxyglucose positron emission tomography in the detection of colorectal metastases. J Clin Oncol 2005;23:8713-6. [PubMed]
- Kulemann V, Schima W, Tamandl D, et al. Preoperative detection of colorectal liver metastases in fatty liver: MDCT or MRI? Eur J Radiol 2011;79:e1-6. [PubMed]
- Kenis C, Deckers F, De Foer B, et al. Diagnosis of liver metastases: can diffusion-weighted imaging (DWI) be used as a stand alone sequence? Eur J Radiol 2012;81:1016-23. [PubMed]
- Taouli B, Sandberg A, Stemmer A, et al. Diffusion-weighted imaging of the liver: comparison of navigator triggered and breathhold acquisitions. J Magn Reson Imaging 2009;30:561-8. [PubMed]
- Bruegel M, Holzapfel K, Gaa J, et al. Characterization of focal liver lesions by ADC measurements using a respiratory triggered diffusion-weighted single-shot echo-planar MR imaging technique. Eur Radiol 2008;18:477-85. [PubMed]
- Bruegel M, Gaa J, Waldt S, et al. Diagnosis of hepatic metastasis: comparison of respiration-triggered diffusion-weighted echo-planar MRI and five t2-weighted turbo spin-echo sequences. AJR Am J Roentgenol 2008;191:1421-9. [PubMed]
- Parikh T, Drew SJ, Lee VS, et al. Focal liver lesion detection and characterization with diffusion-weighted MR imaging: comparison with standard breath-hold T2-weighted imaging. Radiology 2008;246:812-22. [PubMed]
- Holzapfel K, Eiber MJ, Fingerle AA, et al. Detection, classification, and characterization of focal liver lesions: Value of diffusion-weighted MR imaging, gadoxetic acid-enhanced MR imaging and the combination of both methods. Abdom Imaging 2012;37:74-82. [PubMed]
- Zech CJ, Herrmann KA, Reiser MF, et al. MR imaging in patients with suspected liver metastases: value of liver-specific contrast agent Gd-EOB-DTPA. Magn Reson Med Sci 2007;6:43-52. [PubMed]
- Hammerstingl R, Huppertz A, Breuer J, et al. Diagnostic efficacy of gadoxetic acid (Primovist)-enhanced MRI and spiral CT for a therapeutic strategy: comparison with intraoperative and histopathologic findings in focal liver lesions. Eur Radiol 2008;18:457-67. [PubMed]
- Leen E, Ceccotti P, Moug SJ, et al. Potential value of contrast-enhanced intraoperative ultrasonography during partial hepatectomy for metastases: an essential investigation before resection? Ann Surg 2006;243:236-40. [PubMed]
- Fioole B, de Haas RJ, Wicherts DA, et al. Additional value of contrast enhanced intraoperative ultrasound for colorectal liver metastases. Eur J Radiol 2008;67:169-76. [PubMed]
- Torzilli G, Botea F, Donadon M, et al. Criteria for the selective use of contrast-enhanced intra-operative ultrasound during surgery for colorectal liver metastases. HPB (Oxford) 2014. [Epub ahead of print].
- Torzilli G, Del Fabbro D, Palmisano A, et al. Contrast-enhanced intraoperative ultrasonography during hepatectomies for colorectal cancer liver metastases. J Gastrointest Surg 2005;9:1148-53; discussion 1153-4. [PubMed]
- Takahashi M, Hasegawa K, Arita J, et al. Contrast-enhanced intraoperative ultrasonography using perfluorobutane microbubbles for the enumeration of colorectal liver metastases. Br J Surg 2012;99:1271-7. [PubMed]
- Arita J, Ono Y, Takahashi M, et al. Usefulness of contrast-enhanced intraoperative ultrasound in identifying disappearing liver metastases from colorectal carcinoma after chemotherapy. Ann Surg Oncol 2014;21 Suppl 3:S390-7. [PubMed]
- Takahashi M, Hasegawa K, Arita J, et al. Contrast-enhanced intraoperative ultrasonography using perfluorobutane microbubbles for the enumeration of colorectal liver metastases. Br J Surg 2012;99:1271-7. [PubMed]
- Zalinski S, Abdalla EK, Mahvash A, et al. A marking technique for intraoperative localization of small liver metastases before systemic chemotherapy. Ann Surg Oncol 2009;16:1208-11. [PubMed]
- Mahvi DM, Lee FT Jr. Radiofrequency ablation of hepatic malignancies: is heat better than cold? Ann Surg 1999;230:9-11. [PubMed]
- Nahum Goldberg S, Dupuy DE. Image-guided radiofrequency tumor ablation: challenges and opportunities--part I. J Vasc Interv Radiol 2001;12:1021-32. [PubMed]
- Kingham TP, Karkar AM, D’Angelica MI, et al. Ablation of perivascular hepatic malignant tumors with irreversible electroporation. J Am Coll Surg 2012;215:379-87. [PubMed]
- Sakamoto T. Roles of universal three-dimensional image analysis devices that assist surgical operations. J Hepatobiliary Pancreat Sci 2014;21:230-4. [PubMed]