Small for size liver remnant following resection: prevention and management
Introduction
In the latest decades an important change was registered in liver surgery, related to the progress of surgical techniques, anesthesiology and postoperative treatment, allowing a sharp decrease in mortality and morbidity. However, management of liver cirrhosis or small size hepatic remnant still remains a challenge (1).
The liver presents regenerative capacity, allowing performance of repeated resections. In certain cases, when this capacity is impaired, or where extensive resections were performed with small remnant liver, these patients may develop small for size syndrome (SFSS) with the presence of reduced liver mass insufficient to maintain normal liver function.
The term SFSS was first employed in liver transplantation to describe the development of acute liver failure (ALF) (hyperbilirubinemia, coagulopathy, encephalopathy and refractory ascites) resulted from the transplantation of a donor liver that was too small for a given recipient (2). A similar syndrome, called ‘‘post-hepatectomy liver failure (PLF)’’ was also described in hepatic surgery involving extended resections of liver mass. The last one is characterized by postoperative liver dysfunction, with clinical signs of prolonged cholestasis, coagulopathy, portal hypertension and ascites. PLF is the major cause of death after liver resection often associated with sepsis and ischemia-reperfusion injury (IRI) (3).
The patho-physiological mechanisms of the SFSS and PLF are very similar, both presenting reduction of liver mass and portal hyper flow beyond a certain threshold (4).
The aim of this review is to discuss applicable peri-operative methods to prevent the SFSS or PLF and highlight the main treatment types.
Pathophysiology
The liver should contain minimum amount of parenchymal hepatic cells to assure its functions and the maintenance of its regeneration capacity. The hepatic parenchyma should be able to accommodate the hemodynamic changes that occur after liver resection, avoiding venous congestion. Factors such as decrease of hepatic parenchyma cells, infection and different causes that might jeopardize regeneration should be absent (5).
Decrease in parenchymal volume results in a hyper perfusion of the liver, causing dilation of sinusoids, hemorrhagic infiltration, shear stress, centro lobular necrosis, prolonged cholestasis impaired synthetic function and inhibition of cell proliferation (6).
Hepatic resections have higher risks of infection (above 50%). The number of Kupffer cells after hepatic resection decreased and thus the liver’s ability to fight against infection as well. The sepsis possesses the ability to complicate or precipitate PLF. A relative increase in the production of endotoxins in the remnant liver is beneficial, once it activates the Kupffer cells, trigging the liver regeneration. This prolonged state may cause Kupffer’s cellular dysfunction, resulting in difficulty of regeneration and even liver necrosis (7).
The parenchymal damage occurs following vascular occlusion or after hemorrhagic shock, causing IRI. After a period of ischemia, the complement cascade is triggered, leading to the activation of Kupffer cells, reactive oxygen appearance of species (ROS) and endothelial cell lesion. During reperfusion a release of cytokines, cell adhesion, activation and recruitment of T cell and polymorphonuclear cell occurs, resulting in microvascular lesion, inflammation and cell death (8).
Preoperative period-prevention
Liver function tests and scores (9)
The liver function tests can be divided into three types:
Conventional tests, i.e., serum bilirubin, albumin, alkaline phosphatase, gamma glutamyl transpeptidase, prothrombin time (PT) and platelet count;
Quantitative tests, i.e., aminopyrine breath test, antipyrine clearance, caffeine clearance, lidocaine clearance, methacetin breath test, galactose elimination capacity, low-dose galactose clearance, clearance sorbitol, indocyanine green disappearance, albumin synthesis, urea synthesis and 99mTc-GSA;
Scores, i.e., Child-Turcotte-Pugh and MELD.
One of the best tests today to check liver function before surgery is liver retention of indocyanine green. Widely used since the decade of the 70 in Asian countries, and not yet widespread in the west.
Based on the decisional tree [established by Seyama et al. (Figure 1)] identify before the operation which hepatic volume can be resected in cirrhotic patients depending on their liver function (9).
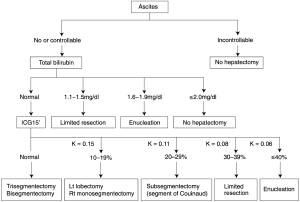
Liver volume (LV) manipulation and liver parenchymal protection
The ideal volume of the hepatic remnant was exhaustively discussed in the literature and some formulas to calculate it were described (10) (Table 1).

Full table
The radiological examinations (mainly CT and/or MRI) before surgery are fundamental to quantify the LV. More recently 3D computed tomography reconstructions could define more accurately the hepatic volume allowing preoperative studies. Through this exam, the surgeon can simulate a resection, making possible the planning and the choice of the best way to do the procedure (15,18).
Measurement of volume ratios correlated with the etiology and severity of chronic liver disease (CLD) constitute a reliable predictors of patient survival (19). Although, the reliability of this ratio might be compromised by the presence of dilated bile ducts, multiple tumors, undetected lesions. Additionally, due to cholestasis or previous chemotherapy, cholangitis, vascular obstruction, steatosis or cirrhosis, or segmental atrophy and/or hypertrophy from tumor growth, negatively impacts the liver function (16).
Values calculated from graft weight-to-recipient body weight ratio (GRBWR), or standardized liver volume (SLV) based on recipient body surface area (BSA) are used to predict minimum adequate graft volume (15). But in presence of steatosis, particularly >30%, graft weight alone is not a suitable guide (10).
Extended resection of 80% of functional parenchyma can be performed in the absence of CLD for hepatobiliary malignancies (20). Recommended minimal functional remnant LV following extended hepatectomy is 25% in a normal liver, and 40% in a “sick” liver, with moderate to severe steatosis, cholestasis, fibrosis, cirrhosis, or following chemotherapy (15).
There are some strategies that allow volume manipulation, such as portal vein embolization (PVE) and two-stage hepatectomy (16,21). PVE is usually performed percutaneously by transhepatic PVE, but may also be achieved by surgical ligation and injection of alcohol or others products to prevent the recanalization of the portal vein. PVE increases the functional capacity of the liver remnant and can increase contralateral lobe volume by up to 20 per cent, with the peak in growth occurring within 2-4 weeks (22).
Patients, in which the liver does not have a good result after PVE are selected as no good candidates for large resections due to the difficulty of regeneration (22). Patients with bilateral tumors when proceeding PVE may stimulate of neoplastic cell growth in the non-embolized lobes, in this cases surgical treatment or ablation [radiofrequency (23,24), microwave (25) and NanoKnife® (personal experience)] of such lesions prior to the embolization are required (26). Neoadjuvant chemotherapy (27) and intra-arterial chemotherapy (28) also can be used in combination with PVE to control tumour load before resection (20,29).
Patients with bilateral liver lesions, where resection is not feasible under one procedure, the two stage hepatectomy is applied, allowing the remaining liver to be resected to achieve the suitable LV at the second stage.
Intraoperative period-prevention
In order to limit parenchymal damage and optimize regenerative capacity, two hepatoprotective measures may be employed: intermittent portal clamping and hypothermic liver preservation.
Intermittent portal clamping with intervals allowed for reperfusion is preferred to continuous clamping, usually applying a 15-min clamp-5-min release regimen (30-32).
Total vascular exclusion of the liver should be used when we have no choice to do the resection without it. When chosen, we can utilized hepatic vascular exclusion with preservation of the caval flow (33).
Hypothermic liver preservation in conjunction with total vascular exclusion attenuates IRI. The future remnant is infused with a preservative fluid and surrounded by crushed ice to maintain the liver at 4 °C. This approach is a useful adjunct to complex resections when total vascular exclusion and vascular reconstructions are programmed (34). During surgery it is still possible to apply techniques to prevent the SFSS, if other procedures were not considered on the pre-operative period.
Association liver partition and portal vein ligation (ALPPS)
ALPPS, a newer strategy to increase resectability of hepatic malignancies, has been described for the first time in 2010 (35). This method relies on the fact (proved in clinical trials) that any closure of portal branch will be followed by a reactive perfusion through intrahepatic branches and collaterals present between two lobes. Hence, partition of the liver along the falciform ligament line, for example, will enhance regeneration compared to traditional methods. ALPPS has shown high hypertrophy rates compared to PVE/PVL (40% to 80% within a week compared to 8% to 27% within 2 to 60 days by PVL/PVE), however it is associated with high morbidity rates (16-64% of patients) and mortality rates (12-23% of patients), therefore a careful selection of surgical candidates should be done prior to surgery. Further investigation if ALPPS approach accelerates tumor growth is still required (35-37).
Recently, a number of comparisons between ALLPS and standard methods (PVE followed by liver resections) have been published (38-40). One of the proposed benefits of ALLPS, for example, is rapid removal of tumor(s), thus preventing patient dropout due to disease progression of existing liver tumors. This assumption, however, failed to achieve clinical relevance in a recent publication that compared right PVE + segment 4 to ALPPS, demonstrating mainly extra-hepatic location of metastasis in the patient’s drop-out group. In addition, using PVE in this study yielded sufficient growth in 96.5% of the patients, with median hypertrophy of 62%, comparable to the FLR hypertrophy rates associated with the ALLPS approach (38).
Although none of the studies published with this technique provide measurements of portal pressure or portal blood flow, the clinical data suggest that the acceleration of the hypertrophy of the residual parenchyma occurs due to the reduction of intra-hepatic communicants, once the in situ split procedure leads to complete portal devascularization of segment 4, preventing formation of collaterals between the left and the right liver that could otherwise undermine the completeness of right portal vein occlusion alone (41).
A second and not mutually exclusive explanation would be the ‘‘regenerating liver’’ hypothesis proposed by Nagano et al. (42).
Modulation of portal pressure
Intraoperative Doppler ultrasonography has been used in combination with hepatic portal inflow modulation to detect and offset hyperperfusion in a small-for-size graft. Importantly, numerous interventions that modulate the portal blood flow have been shown to prevent the development of the SFSS in experimental models, such as: the performance of a portocaval anastomosis (43,44), the ligation of the splenic artery (45), banding of the portal vein (46) or the infusion of adenosine (47), somatostatin (48), pentoxifylline (49) or endothelin-1 (50). It is important to highlight that the role for inflow modulation at the time of major liver resection or as a salvage therapy in humans remains undefined.
After all these studies cited above we can conclude that the development of SFSS or PLF are not strictly determined by the ‘‘size’’ of the liver graft or remnant. It is determined by the hemodynamic parameters of the hepatic circulation and, specifically, by a portal blood flow that, when excessive for the volume of the liver parenchyma leads to over-pressure, sinusoidal endothelial denudation and hemorrhage. Perisinusoidal and periportal hemorrhage occurs in the first minutes after an extended hepatic resection as well as after the reperfusion of a small graft, while arterial vasoconstriction and ischemic cholangitis are observed at later stages (6).
Also, experimental and clinical studies consistently show that an increased portal blood flow relative to the weight of the liver results in an inverse relationship between portal and arterial blood flows that is known as the arterio-portal buffer (51). The arterio-portal buffer occurs when the portal blood flow increases, the adenosine concentration in the space of mall decreases leading to arterial vasoconstriction and decrease of arterial blood flow, which is responsible for the late damage (52).
Studies performed in patients undergoing liver transplantation in which the portal and hepatic arterial blood flows were measured intra-operatively have provided further insights into the pathophysiology of the SFSS (6,53,54). A portal blood flow of 300 mL/min/100 g was established by Jiang et al. as the threshold above that the incidence of the SFSS increases significantly (54).
In living donor liver transplantations involving grafts with GWRW below 0.8, Troisi et al. showed that the construction of a portal-systemic shunt whenever the portal blood flow exceeded 250 mL/min/100 g was able to prevent the histological alterations characteristic of the SFSS and to improve the overall patient and graft survival (43,54).
Several studies indicate that additionally to blood flow, portal pressure can also be considered a good parameter for predicting the failure of the graft. For example, patients with a portal pressure higher than 20 mmHg show a decrease from 85% to 38% in their 6-month survival (55). Yagi et al. also described that a portal pressure above 20 mmHg was associated with the development of ascites, coagulopathy and hyperbilirubinemia as well as with an early hypertrophy of the graft, higher values of hepatocyte growth factor (HGF) and diminished levels of vascular epithelial growth factor (VEGF), suggesting that an increased portal pressure also influences liver regeneration (56). Kaido et al. reported their experience with small grafts (GWRW of 0.6) in combination with portal pressure control (targeting final portal pressures below 15 mmHg), showing that the survival of recipients of small grafts and standard-size grafts was similar and that the portal pressure control strategy resulted in a decreased rate of complications in the donors (57).
As in liver transplantation, studies involving extended hepatic resections also indicate that the increased portal blood flow with diminished residual parenchyma are a critical factor determining the development of PLF (47,58,59). The performance of a portocaval anastomosis in a patient with liver cirrhosis undergoing a major hepatectomy effectively prevented the syndrome, probably by reducing shear stress and damage to the sinusoids (60).
Post-operative period-treatment (61)
PLF is a quite complex disease, that requires a multi-disciplinary approach, where it management must be undertaken in conjunction with critical care, hepatology, microbiology and radiology services (1).
After liver resection, clinical and laboratory assessment should be proceeded. Normally, the level of serum bilirubin and the INR rises in the first 48-72 h after resection. It is possible to identify liver dysfunction, whenever bilirubin concentration is above 50 µmol/L (3 mg/dL) or INR greater than 1.7 beyond 5 days of surgery (3). The most sensitive variable is serum bilirubin as predictor of outcome in PLF (62). PT and INR are also relevant, but the interpretation may be compromised if patients have received clotting factors.
Serum albumin, although an indicator of hepatic synthetic function, will vary in response to inflammation and administration of intravenous fluids (63,64). Increased levels of liver enzymes are common after liver resection and do not predict outcome (3).
Ascites and hepatic encephalopathy are important markers for liver failure, although it may be difficult to assess in the immediate postoperative period. The first occurs as a result of surgery (portal hypertension, dissection, gross fluid overload), while the second is a result of mental state as collateral effect of drugs such as opiates (62).
Several studies assessed the role of postoperative functional of the liver. This task still consist a challenge, once the ICGR15 is capable to predicts PLF (65), but its value diminishes once liver failure is established, since the changes in hepatic blood flow impacts ICGR15. In the absence of controlled trials for PLF, management relies on data from experience with ALF, secondary to paracetamol toxicity (66-68).
The pattern of organ dysfunction that occurs as a result of PLF is similar to that in sepsis (1). Once the following symptoms occur: cardiovascular failure, characterized by reduced systemic vascular resistance and capillary leak; acute lung injury, due to pulmonary edema and acute respiratory distress syndrome may ensue and acute kidney injury can progress rapidly in PLF. In those cases, fluid balance should be managed judiciously with avoidance of salt and water overload (64). Identifying and treating underlying sepsis is key in managing patients with PLF. Sepsis may exacerbate PLF, and bacterial infection is present in 80 per cent of patients with PLF (69) and in 90 per cent of those with ALF (70).
Therefore, any acute deterioration should be attributed to sepsis until proven otherwise. Management of sepsis should be in accordance with the surviving sepsis guidelines (71). A trial of prophylactic antibiotics after liver resection failed to show a reduction in liver dysfunction or infective complications (72). A study of ALF have shown that prophylactic antibiotics reduce infections, but the impact on a long-term outcome is inconclusive (70). In critically ill patients with PLF, chest radiography and cultures of blood, urine, sputum and drain site/ascitic fluid should be performed (68). Current guidelines for ALF propose that broad-spectrum antibiotics should be administered empirically to patients with progression to grade 3 or 4 of hepatic encephalopathy, renal failure and/or worsening SIRS parameters (68).
Additionally coagulopathy may occur transiently after major resection and is found in all patients with PLF. As in ALF, coagulation parameters can be used to chart the progress of PLF, provided blood products have not been given. In the absence of bleeding it is not necessary to correct clotting abnormalities, except for invasive procedures or when coagulopathy is severe. The level at which a coagulopathy should be corrected before an interventional procedure in ALF has yet to be defined (66,68,73). Vitamin K may be given, but this is not supported by clinical trials (66). Thrombocytopenia may complicate liver failure (74). Indications for platelet transfusion in ALF include bleeding, severe thrombocytopenia (less than 20×106/L), or when an invasive procedure is planned. A platelet count above 70×106/L is deemed safe for interventional procedures (75). Recombinant factor VIIa (rFVIIa) has been used to treat coagulopathy in patients with ALF (76). In a large controlled trial of rFVIIa following major liver resection, no reduction in bleeding events was observed (77). Its role in PLF is yet to be defined.
Gastrointestinal hemorrhage is a recognized complication of liver failure. In ALF, H2-receptor blockers and proton pump inhibitors (PPIs) reduce gastrointestinal ill patients ensuring euglycemia improves survival and reduces morbidity (78).
The role of imaging in PLF is to assess hepatic blood flow, identify reversible causes of liver failure and locate sites of infection. Hepatic blood flow can be evaluated using non-invasive imaging. Doppler ultrasonography may identify portal vein, hepatic artery and hepatic vein thrombosis. Contrast CT or MRI can be used to establish hepatic blood flow, provide more details of vascular abnormalities and identify sites of infection. If patency of hepatic vessels is still in doubt on cross-sectional imaging, angiography is the “gold standard” (79).
Portal vein thrombosis has also been implicated in the development of PLF. In these rare cases of inflow and outflow thrombosis with PLF, a decision must be taken regarding the benefit of surgical or radiological thrombectomy or dissolution versus anticoagulation (80,81). The use of terlipressin also can reduce the portal venous pressure helping to hepatic regeneration (82). Cerebral edema and intracranial hypertension may occur as a result of PLF. It is unlikely in patients with grade 1 or 2 of liver encephalopathy. When achieving grade 3 encephalopathy, a head CT should be performed to exclude intracranial hemorrhage or other causes of declining mental status.
In patients with established ALF and encephalopathy, enteral lactulose might prevent or treat cerebral edema, although the benefits remain unproven. Progression to grade 3/4 encephalopathy warrants ventilation and may require intracranial pressure monitoring (68).
The concept of hepatocyte transplantation has been investigated as a strategy to boost residual liver function. Intrahepatic hepatocyte transplantation (83) has been used successfully to treat patients with metabolic disorders of the liver. However, results in liver failure (including patients with PLF) have been poor due to insufficient delivery of functional cells. The potential for stem cell therapies has yet to be established (84).
The use of salvage hepatectomy and orthotopic liver transplantation for PLF has been reported in seven patients who underwent liver resection for cancer (85). Although the indications for transplantation in this study were questionable, overall 1-year (88 per cent) and 5-year (40 per cent) survival rates were promising.
Extracorporeal liver support (ELS) devices fall into two categories: artificial and bioartificial systems. Artificial devices use combinations of haemodialysis and adsorption over charcoal or albumin to detoxify plasma. Bioartificial devices use human or xenogenic hepatocytes maintained within a bioreactor to detoxify and provide synthetic function. These systems have not been evaluated extensively in patients with PLF. A recent meta-analysis and systematic review showed that ELS may improve survival in patients with ALF, but not acute-on-chronic liver failure, in comparison with standard medical therapy (86).
Conclusions
The increased use of small liver grafts and the expansion of indications of curative liver surgery in patients with hepatic tumors allows a step change in the knowledge of the mechanisms responsible for the development of the SFSS and PLF.
It became evident that the size of the liver cannot be considered the main variable in the development of liver dysfunction after extended hepatectomies. Additional characteristics should be taken into account, such as: the future liver remnant; the portal blood flow and pressure and the exploration of the potential effects of regeneration preconditioning are all promising strategies that could help to expand the indications and increase the safety of liver surgery.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- van den Broek MA, Olde Damink SW, Dejong CH, et al. Liver failure after partial hepatic resection: definition, pathophysiology, risk factors and treatment. Liver Int 2008;28:767-80. [PubMed]
- Ikegami T, Shimada M, Imura S, et al. Current concept of small-for-size grafts in living donor liver transplantation. Surg Today 2008;38:971-82. [PubMed]
- Balzan S, Belghiti J, Farges O, et al. The 50-50 Criteria on Postoperative Day 5. Ann Surg 2005;242:824-8, discussion 828-9. [PubMed]
- Asencio JM, Vaquero J, Olmedilla L, et al. “Small-for-flow” syndrome: shifting the “size” paradigm. Med Hypotheses 2013;80:573-7. [PubMed]
- Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology 2006;43:S45-53. [PubMed]
- Demetris AJ, Kelly DM, Eghtesad B, et al. Pathophysiologic Observations and Histopathologic Recognition of the Portal Hyperperfusion or Small-for-size-syndrome. Am J Surg Pathol 2006;30:986-93. [PubMed]
- Panis Y, McMullan DM, Emond JC. Progressive necrosis after hepatectomy and the pathophysiology of liver failure after massive resection. Surgery 1997;121:142-9. [PubMed]
- Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol 2003;284:G15-26. [PubMed]
- Seyama Y, Kokudo N. Assessment of liver function for safe hepatic resection. Hepatol Res 2009;39:107-16. [PubMed]
- Tucker ON, Heaton N. The “small for size” liver syndrome. Curr Opin Crit Care 2005;11:150-5. [PubMed]
- Urata K, Kawasaki S, Matsunami H. Calculation of child and adult standard liver volume for liver transplantation. Hepatology 1995;21:1317-21. [PubMed]
- Lin XZ, Sun Y, Liu Y, et al. Liver volume in patients with or without chronic liver diseases. Hepatogastroenterology 1998;45:1069-74. [PubMed]
- Heinemann A, Wischhusen F, Püschel K, et al. Standard liver volume in the Caucasian population. Liver Transpl Surg 1999;5:366-8. [PubMed]
- Vauthey JN, Abdalla EK, Doherty DA, et al. Body surface area and body weight predict total liver volume in Western adults. Liver Transpl 2002;8:233-40. [PubMed]
- Vauthey JN, Chaoui A, Do KA, et al. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery 2000;127:512-9. [PubMed]
- Azoulay D, Castaing D, Smail A, et al. Resection of nonresectable liver metastases from colorectal cancer after percutaneous portal vein embolization. Ann Surg 2000;231:480-6. [PubMed]
- Abdalla EK, Denys A, Chevalier P, et al. Total and segmental liver volume variations: implications for liver surgery. Surgery 2004;135:404-10. [PubMed]
- Mise Y, Tani K, Aoki T, et al. Virtual liver resection: computer-assisted operation planning using a three-dimensional liver representation. J Hepatobiliary Pancreat Sci 2013;20:157-64. [PubMed]
- Zhou XP, Lu T, Wei Y, et al. Liver volume variation in patients with virus-induced cirrhosis: findings on MDCT. AJR Am J Roentgenol 2007;189:W153-9. [PubMed]
- Abdalla EK, Barnett CC, Doherty D, et al. Extended hepatectomy in patients with hepatobiliary malignancies with and without preoperative portal vein embolization. Arch Surg 2002;137:675-80; discussion 680-1. [PubMed]
- Adam R, Laurent A, Azoulay D, et al. Two-stage hepatectomy: A planned strategy to treat irresectable liver tumors. Ann Surg 2000;232:777-85. [PubMed]
- Farges O, Belghiti J. Portal Vein Embolization Before Right Hepatectomy Prospective clinical trial. Ann Surg 2003;237:208-17. [PubMed]
- Elias D, Santoro R, Ouellet J. Simultaneous percutaneous right portal vein embolization and left liver tumor radiofrequency ablation prior to a major right hepatic resection for bilateral colorectal metastases. Hepatogastroenterology 2004;51:1788. [PubMed]
- Akgül Ö, Çetinkaya E, Ersöz Ş, et al. Role of surgery in colorectal cancer liver metastases. World J Gastroenterol 2014;20:6113-22. [PubMed]
- Bala MM, Riemsma RP, Wolff R, et al. Microwave coagulation for liver metastases. Cochrane Database Syst Rev 2013;10:CD010163. [PubMed]
- Hoekstra LT, van Lienden KP, Doets A, et al. Tumor progression after preoperative portal vein embolization. Ann Surg 2012;256:812-7; discussion 817-8. [PubMed]
- Adam R, Delvart V, Pascal G, et al. Rescue Surgery for Unresectable Colorectal Liver Metastases Downstaged by Chemotherapy. A model to predict long term survival. Ann Surg 2004;240:644-57. [PubMed]
- Selzner N, Pestalozzi BC, Kadry Z, et al. Downstaging colorectal liver metastases by concomitant unilateral portal vein ligation and selective intra-arterial chemotherapy. Br J Surg 2006;93:587-92. [PubMed]
- Wicherts DA, Miller R, de Haas RJ, et al. Long-Term Results of Two-Stage Hepatectomy for Irresectable Colorectal Cancer Liver Metastases. Ann Surg 2008;248:994-1005. [PubMed]
- Brooks AJ, Hammond JS, Girling K, et al. The effect of hepatic vascular inflow occlusion on liver tissue pH, carbon dioxide, and oxygen partial pressures: defining the optimal clamp/release regime for intermittent portal clamping. J Surg Res 2007;141:247-51. [PubMed]
- Belghiti J, Noun R, Malafosse R. Continuous Versus Intermittent Portal Triad Clamping for Liver Resection. A Controlled study. Ann Surg 1999;229:369-75. [PubMed]
- Belghiti J, Noun R, Zante E. Portal Triad Clamping or Hepatic Vascular Exclusion for Major Liver Resection. A controlled study. Ann Surg 1996;224:155-61. [PubMed]
- Cherqui D, Malassagne B, Colau P. Hepatic Vascular Exclusion With Preservation of the Caval Flow for Liver Resections. Ann Surg 1999;230:24-30. [PubMed]
- Azoulay D, Eshkenazy R, Andreani P, et al. In situ hypothermic perfusion of the liver versus standard total vascular exclusion for complex liver resection. Ann Surg 2005;241:277-85. [PubMed]
- Schnitzbauer AA, Lang SA, Goessmann H, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg 2012;255:405-14. [PubMed]
- Andriani OC. Long-term results with associating liver partition and portal vein ligation for staged hepatectomy (ALPPS). Ann Surg 2012;256:e5; author reply e16-9.
- de Santibañes E, Clavien PA. Playing Play-Doh to prevent postoperative liver failure: the “ALPPS” approach. Ann Surg 2012;255:415-7. [PubMed]
- Shindoh J, Vauthey JN, Zimmitti G, et al. Analysis of the efficacy of portal vein embolization for patients with extensive liver malignancy and very low future liver remnant volume including a comparison to the ALPPS approach. J Am Coll Surg 2014;217:126-33; discussion 133-4. [PubMed]
- Tschuor C, Croome KP, Sergeant G, et al. Salvage parenchymal liver transection for patients with insufficient volume increase after portal vein occlusion -- an extension of the ALPPS approach. Eur J Surg Oncol 2013;39:1230-5. [PubMed]
- Schadde E, Ardiles V, Slankamenac K, et al. ALPPS offers a better chance of complete resection in patients with primarily unresectable liver tumors compared with conventional-staged hepatectomies: results of a multicenter analysis. World J Surg 2014;38:1510-9. [PubMed]
- Chan A, Chung PH, Poon RT. Little girl who conquered the “ALPPS”. World J Gastroenterol 2014;20:10208-11. [PubMed]
- Nagano Y, Nagahori K, Kamiyama M, et al. Improved functional reserve of hypertrophied contra lateral liver after portal vein ligation in rats. J Hepatol 2002;37:72-7. [PubMed]
- Troisi R, Ricciardi S, Smeets P, et al. Effects of hemi-portocaval shunts for inflow modulation on the outcome of small-for-size grafts in living donor liver transplantation. Am J Transplant 2005;5:1397-404. [PubMed]
- Takada Y, Ueda M, Ishikawa Y, et al. End-to-side portocaval shunting for a small-for-size graft in living donor liver transplantation. Liver Transpl 2004;10:807-10. [PubMed]
- Luca A, Miraglia R, Caruso S. Effects of splenic artery occlusion on portal pressure in patients with cirrhosis and portal hypertension. Liver Transpl 2006;12:1237-43. [PubMed]
- Reyal J, Uemoto S. Percutaneously adjustable portal vein banding device could prevent post-operative liver failure--artificial control of portal venous flow is the key to a new therapeutic world. Med Hypotheses 2009;73:640-50. [PubMed]
- Kelly DM, Zhu X, Shiba H, et al. Adenosine restores the hepatic artery buffer response and improves survival in a porcine model of small-for-size syndrome. Liver Transpl 2009;15:1448-57. [PubMed]
- Matrella E, Valatas V, Notas G, et al. Bolus somatostatin but not octreotide reduces hepatic sinusoidal pressure by a NO-independent mechanism in chronic liver disease. Aliment Pharmacol Ther 2001;15:857-64. [PubMed]
- Tian Y, Jochum W. Kupffer cell-dependent TNF- signaling mediates injury in the arterialized small-for-size liver transplantation in the mouse. PNAS 2006;103:4598-603. [PubMed]
- Kawachi S, Shimazu M. Efficacy of intraportal infusion of prostaglandin E1 to improve the hepatic blood flow and graft viability in porcine liver transplantation. Transplantation 1997;64:205-9. [PubMed]
- Eipel C. Regulation of hepatic blood flow: The hepatic arterial buffer response revisited. World J Gastroenterol 2010;16:6046. [PubMed]
- Fondevila C, Hessheimer A. Portal hyperperfusion: Mechanism of injury and stimulus for regeneration in porcine small-for-size transplantation. Liver Transpl 2010;16:364-74. [PubMed]
- Troisi R, de Hemptinne B. Clinical relevance of adapting portal vein flow in living donor liver transplantation in adult patients. Liver Transpl 2003;9:S36-41. [PubMed]
- Jiang SM, Zhou G, Zhang R. Role of splanchnic hemodynamics in liver regeneration after living donor liver transplantation. Liver Transpl 2009;15:1043-9. [PubMed]
- Boillot O, Delafosse B, Méchet I, et al. Small-for-size partial liver graft in an adult recipient; a new transplant technique. Lancet 2002;359:406-7. [PubMed]
- Yagi S, Iida T, Hori T, et al. Optimal portal venous circulation for liver graft function after living-donor liver transplantation. Transplantation 2006;81:373-8. [PubMed]
- Kaido T, Mori A, Ogura Y, et al. Lower limit of the graft-to-recipient weight ratio can be safely reduced to 0.6% in adult-to-adult living donor liver transplantation in combination with portal pressure control. Transplant Proc 2011;43:2391-3. [PubMed]
- Hessheimer AJ, Fondevila C, Taurá P, et al. Decompression of the portal bed and twice-baseline portal inflow are necessary for the functional recovery of a “small-for-size” graft. Ann Surg 2011;253:1201-10. [PubMed]
- Farantos C, Arkadopoulos N, Theodoraki K, et al. Effect of the portacaval shunt on reperfusion injury after 65% hepatectomy in pigs. Eur Surg Res 2008;40:347-53. [PubMed]
- Polacco M, Vitale A, Valmasoni M, et al. Liver resection associated with mini porto-caval shunt as salvage treatment in patients with progression of hepatocellular carcinoma before liver transplantation: a case report. Transplant Proc 2010;42:1378-80. [PubMed]
- Hammond JS, Guha IN, Beckingham IJ, et al. Prediction, prevention and management of postresection liver failure. Br J Surg 2011;98:1188-200. [PubMed]
- Mullen JT, Ribero D, Reddy SK, et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg 2007;204:854-62; discussion 862-4. [PubMed]
- Lobo DN, Stanga Z, Aloysius MM, et al. Effect of volume loading with 1 liter intravenous infusions of 0.9% saline, 4% succinylated gelatine (Gelofusine) and 6% hydroxyethyl starch (Voluven) on blood volume and endocrine responses: a randomized, three-way crossover study in healthy volunteers. Crit Care Med 2010;38:464-70. [PubMed]
- Varadhan KK, Lobo DN. A meta-analysis of randomised controlled trials of intravenous fluid therapy in major elective open abdominal surgery: getting the balance right. Proc Nutr Soc 2010;69:488-98. [PubMed]
- de Liguori Carino N, O’Reilly DA, Dajani K, et al. Perioperative use of the LiMON method of indocyanine green elimination measurement for the prediction and early detection of post-hepatectomy liver failure. Eur J Surg Oncol 2009;35:957-62. [PubMed]
- Polson J, Lee WM. AASLD position paper: the management of acute liver failure. Hepatology 2005;41:1179-97. [PubMed]
- Jalan R. Acute liver failure: current management and future prospects. J Hepatol 2005;42 Suppl:S115-23.. [PubMed]
- Stravitz RT, Kramer DJ. Management of acute liver failure. Nat Rev Gastroenterol Hepatol 2009;6:542-53. [PubMed]
- Schindl MJ, Redhead DN, Fearon KCH, et al. The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut 2005;54:289-96. [PubMed]
- Rolando N, Gimson A, Wade J. Prospective controlled trial of selective parenteral and enteral antimicrobial regimen in fulminant liver failure. Hepatology 1993;17:196-201. [PubMed]
- Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013;39:165-228. [PubMed]
- Wu CC, Yeh DC, Lin MC, et al. Prospective randomized trial of systemic antibiotics in patients undergoing liver resection. Br J Surg 1998;85:489-93. [PubMed]
- O’Shaughnessy DF, Atterbury C, Bolton Maggs P, et al. Guidelines for the use of fresh-frozen plasma, cryoprecipitate and cryosupernatant. Br J Haematol 2004;126:11-28. [PubMed]
- Schiødt FV, Balko J, Schilsky M, et al. Thrombopoietin in acute liver failure. Hepatology 2003;37:558-61. [PubMed]
- Drews RE, Weinberger S. Thrombocytopenic disorders in critically ill patients. Am J Respir Crit Care Med 2000;162:347-51. [PubMed]
- Shami VM, Caldwell SH, Hespenheide EE, et al. Recombinant activated factor VII for coagulopathy in fulminant hepatic failure compared with conventional therapy. Liver Transpl 2003;9:138-43. [PubMed]
- Lodge JP, Jonas S. Recombinant coagulation factor VIIa in major liver resection: a randomized, placebo-controlled, double-blind clinical trial. Anesthesiology 2005;102:269-75. [PubMed]
- van Den Berghe G. Intensive insulin therapy in critically ill patients. N Engl J Med 2001;345:1359-67. [PubMed]
- DeLeve LD, Valla DC, Garcia-Tsao G. Vascular disorders of the liver. Hepatology 2009;49:1729-64. [PubMed]
- Adani GL, Baccarani U, Risaliti A, et al. Percutaneous transhepatic portography for the treatment of early portal vein thrombosis after surgery. Cardiovasc Intervent Radiol 2007;30:1222-6. [PubMed]
- Ozeki Y, Umemoto T. Partial portal arterialization for dearterialized liver after hepatectomy. Br J Surg 1997;84:1011. [PubMed]
- Fahrner R, Patsenker E, de Gottardi A, et al. Elevated liver regeneration in response to pharmacological reduction of elevated portal venous pressure by terlipressin after partial hepatectomy. Transplantation 2014;97:892-900. [PubMed]
- Fox IJ, Roy-Chowdhury J. Hepatocyte transplantation. J Hepatol 2004;40:878-86. [PubMed]
- Stutchfield BM, Forbes S, Wigmore S. Prospects for stem cell transplantation in the treatment of hepatic disease. Liver Transpl 2010;16:827-36. [PubMed]
- Otsuka Y, Duffy J, Saab S. Postresection hepatic failure: successful treatment with liver transplantation. Liver Transpl 2007;13:672-9. [PubMed]
- Stutchfield BM, Simpson K, Wigmore SJ. Systematic review and meta-analysis of survival following extracorporeal liver support. Br J Surg 2011;98:623-31. [PubMed]


