Role of ischemic preconditioning in hepatic ischemia-reperfusion injury
Introduction
Inflow hepatic vascular occlusion (Pringle maneuver) is the simple and effective in minimizing bleeding during liver resection (1,2). The resulting hepatic parenchyma ischemia-reperfusion injury affects the condition and functionality of the remaining organ part, which is essential in major resections and in case of diffuse liver diseases (hepatitis, fibrosis, cirrhosis) (1,3-5). Considering this, some surgeons did not use hepatic vascular occlusion. One of the techniques used to attenuate ischemia-reperfusion syndrome (IRS) is ischemic preconditioning (6-9). The goal of our study was to determine in the experiment the optimal model of vascular clamping which causes minimum pathological changes in liver without depressing its regeneration functions.
Materials and methods
Experiment design. Forty white giant rabbits with a body weight between 3.5 and 4.2 kg were used for the experiment. Animals were housed in standard animal laboratories with a constant temperature of 23 °C and a 12 hours light-dark cycle. They had free access to food and water until the start of the experiment. All experiments are conducted according to “the General principles of experiments on animals”, approved by Ethics Committee of the GI “Institute of General and Urgent Surgery of NAMS of Ukraine”, I national congress on bioethics (20.09.2004, Kiev, Ukraine) and compounded with positions “the European convention on protection of vertebrate animals which are used for experiments and other scientific purposes” (Declaration of Helsinki).
Xylazine (5 mg/kg) was injected intramuscularly as premedication intro all rabbits. For the induction of general anesthesia, ketamine (40 mg/kg) was injected also intramuscularly into all rabbits. A midline laparotomy was performed, and hepatic ischaemia was accomplished using a soft vascular clamp, placed on the hepatoduodenal ligament, i.e., the Pringle maneuver.
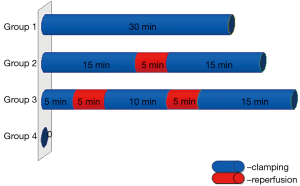
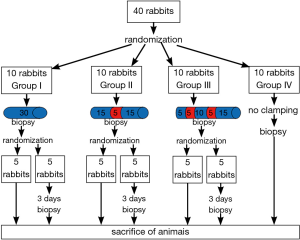
The animals were randomly allocated into groups (ten rabbits per group). In group I was performed continued Pringle maneuver by 30 minutes. In group II was performed intermittent Pringle maneuver: 15 minutes ischemia/5 minutes reperfusion/15 minutes ischemia. In group III was performed intermittent Pringle maneuver with ischemic precondition: 5 minutes ischemia/5 minutes reperfusion/10 minutes ischemia/5 minutes reperfusion/15 minutes ischemia. The duration of vascular clamping was the same in groups (30 minutes). Rabbits in group IV (control group) were operated on in the same way as the other groups using mobilization of the hepatoduodenal ligament, but without clamping it (Figure 1). At the end of each experiment a biopsy was taken from the liver edge. Five rabbits from each group underwent re-laparotomy on day 3 after surgery with biopsy samples being taken for studying reparative processes in liver parenchyma.
Five animals from each group were sacrificed immediately after surgery with thiopental (50 mg/kg intravenously). The other five animals in each group were sacrificed immediately after second surgery with thiopental (50 mg/kg intravenously) (Figure 2).
Liver slides were excised for microscopic examination; they were kept in a 10% neutral formalin solution for 48 hours; histological examinations were performed according to a standard procedure by preparing serial 5-6 µm thick paraffin sections. Plain preparations stained with haematoxylin and eosin was used for general assessment of the examined tissue condition and morphometric examination.
A series of histochemical techniques were used to assess morphofunctional hepatocyte activity: amylase-controlled McManus and Hotchkiss modification of periodic acid Schiff method for glycogen detection; Feulgen-Rossenbeck reaction for desoxyribonucleic acid (DNA) (controlled by hydrolysis with HCl).
Hepatocyte injury grade was assessed using a morphometric technique according to the number and percentage of damaged hepatocytes with moderate functional activity to irreversibly injured cells. Thirty cells in each of 5 random fields of vision were assessed on a microphoto with 400× zoom (150 cells per liver).
The results obtained were processed using mathematical statistics methods. The arithmetic mean, average error (non-sampling error), mean square deviation, relative quantities and average error of relative quantities were calculated. The distribution type in the resulting data arrays was defined. Statistical significance for normal distributions was estimated according to the Student’s criterion. The minimum precision P<0.05 was allowed for estimations of the degree of probability, corresponding to 95% of error-free probability.
Statistical processing of results was performed on PC IBM Acer Extensa 5220 using the statistical software Stat Plus 2009 Professional 5.8.4.
Results
Structural and morphological changes
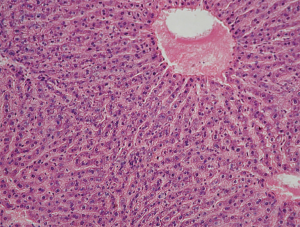
It was observed during the examination of liver samples of group IV animals that cells are organized into segments with venules in the center and hepatic triads consisting of venules, arterioles, lymph vessels and bile ducts on the periphery. The central veins and the sinusoid vessels tree are moderately plethoric. The structure of hepatic lobules was characterized by radial trabecular orientation in all examinations. The hepatocyte cytoplasm was eosinophilic and heterogeneous due to the presence of voids with uneven contours corresponding to glycogen deposits. The nuclei were blue with well-marked karyolemma and clumpy karyoplasm structure (Figure 3).
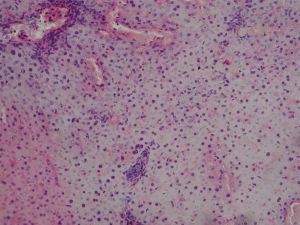
Tissue injury in liver samples of group I animals was characterized by diffuse intertrabecular edema, dilation of perisinusoidal spaces, trabecular decomplexation, diffuse necrobiotic and necrotic hepatocyte changes. The hepatocyte cytoplasm is lightly stained and sharply basophilic (sign of RNA destruction). Karyopyknosis and karyolysis are observed (Figure 4). There are signs of cytolysis in most cells. Unchanged hepatocytes are located in small groups in periportal regions or central sections of lobules, around central veins. In group II, hepatocytes with pronounced edema and dilated perisinusoidal spaces are observed. Sinusoidal vessels are also dilated and congested. Central veins and portal canals are markedly plethoric. Trabecular decomplexation is less pronounced as compared to the 30-minute ischemia group. Heterogeneous hepatic tissue injury is observed: zones with more cells and pronounced alterative changes (necrobiotically changed and necrotizing cells) alternated zones of cells with moderate functional activity and small number of injured cells in a mosaic way (Figure 5). Group III demonstrated mosaic abnormality of organ histoarchitectonics in the form of trabecular decomplexation. Sinusoid vessels are dilated and plethoric. Central veins and portal canals are plethoric. No advanced necrosis lesions are present. Zones with relatively high frequency of unchanged cells with moderate functional activity and small number of dystrophic and necrotizing cells alternate zones with pronounced alterative changes in a mosaic way. Hepatocytes with reversible dystrophic changes, edema and cytoplasm granularity are observed in zones that were not subjected to necrosis. In hydropically changed hepatocytes, hyperchromic nuclei were identified, many of which were in the state of karyopyknosis (signs of irreversibly injured cells) (Figure 6).
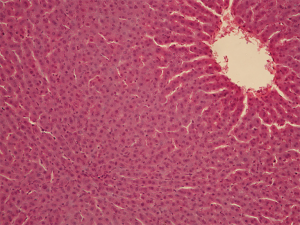
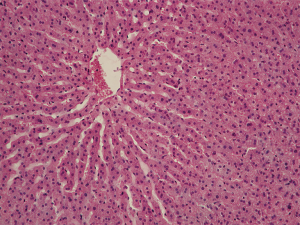
Glycogen
Glycogen was absent or traced in hepatocyte cytoplasm of group I animals (Figure 7), which is indicative of a considerable decrease in morphofunctional cell activity. Only occasional hepatocytes demonstrated light crimson red diffuse cytoplasm staining. There were a small number of fine glycogen granules in the cytoplasm of Kupffer’s cells indicating their high resistance to hypoxia. Glycogen was almost absent in group II, with only traces being detected in the hepatic tissue in the form of scarce diffuse crimson red cytoplasm staining. Clumpy glycogen depositing in hepatocyte cytoplasm of most hepatic lobules were moderately stained in group III, which was indicative of the retention of cellular exchange and synthetic processes. The nature and amount of glycogen deposits were analogous to those in group IV (Figure 8).
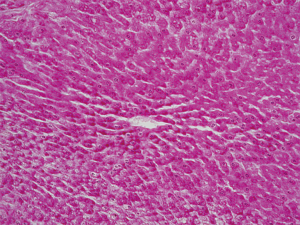
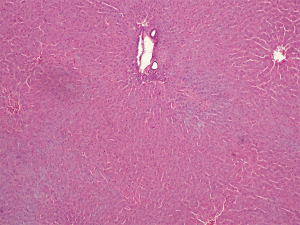
DNA
After staining by the Feulgen-Rossenbeck reaction, there was no staining of hepatocyte nuclei in group I, indicating irreversible cell injury (Figure 9). Hepatocyte nuclei were slightly stained to unstained in group II. Slightly positive to moderate blue and violet staining of hepatocyte nuclei was identified in group III. Kupffer’s cells nuclei were intensively stained (Figure 10).
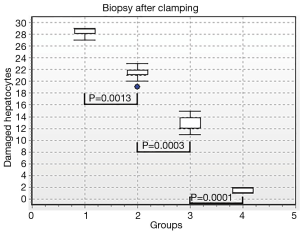
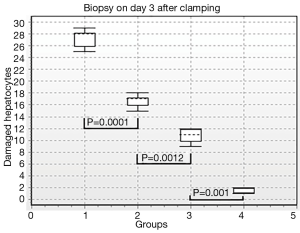
Results of morphometric analysis were the best to illustrate different grades of liver injury in the groups. Thus, there were 95.5% damaged hepatocytes after vascular occlusion in hepatic preparations in group I, 70.3% damaged hepatocytes in group II, and 42.3% damaged hepatocytes in group III. There were 5.3% damaged hepatocytes in the control group (Figure 11). Performance differences between the groups were statistically significant (P1-2=0.0013; P2-3=0.0003; P3-4=0.0001). After 3 days of reperfusion there were 90.6% damaged hepatocytes in hepatic preparations in group I, 55.3% damaged hepatocytes in group II, 36% damaged hepatocytes in group III. There were 5.3% damaged hepatocytes in the control group (Figure 12). Performance differences between the groups were statistically significant (P1-2=0.0001; P2-3=0.0012; P3-4=0.001).
Discussion
By comparing structural hepatic changes in ischemic injuries of different grades it was established that there were similar organ changes in all experimental models (continuous ischemia and ischemia/reperfusion regimens), in particular, dyscirculatory, dystrophic and necrotic processes with macrophages and lymphocytes reacting to them.
Morphological signs of liver parenchyma alteration in case of ischemic injuries included dystrophic changes from granular to vacuolar degeneration, as well as necrotic changes in the form of hepatocyte cytolysis. Glycogen tended to disappear from hepatocyte cytoplasm which is typical of hepatocyte injury. Decrease in PAS-positive products (glycogen) in hepatocytes was strongly oriented—from hepatic triads to the central vein. The earliest and the most pronounced injuries were identified in the periportal region, while the latest and the least pronounced ones were identified in the paracentral zone of the hepatic lobule, therefore dystrophic cellular injury is primarily localized in the periportal region then spreading further to other lobule zones. Thus, injury growth may be traced back from the region with intensive blood flow to the region with less intensive blood supply. Severity and reversibility of injury vary depending on the duration of ischemia and reperfusion periods. Minimum changes were observed in the ischemic injury model in group III with the most pronounced ones being observed in group I. Severity of morphological changes in group II falls in between. Comparative morphological analysis of hepatic structural changes in different ischemia models proved this statement. Hyperplasia of Kupffer’s cells and lymphohistiocytic infiltration of portal canals is a response to injury caused by continues hepatic ischemia. Within the delayed ischemia period (day 3), group III animals, though continuing to show pathological hepatocyte changes, demonstrated signs of their regeneration evidenced by an increase in the number of diploid hepatocytes and their polyploidy, thus indicating presence of liver reparative reserve. Therefore, our study confirms the results of previously conducted studies that showed a protective effect of ischemic precondition for liver resection performed under inflow occlusion (6-9).
Conclusions
Vascular occlusion with ischemic preconditioning in the mode 5/10/15 is based on the most delicate technique that does not involve major structural injuries and functional disorders in the remnant liver. It allows us to recommend it for clinical application as an optimum technique to be applied during liver resection with inflow vascular occlusion.
Acknowledgements
Abstract has been submitted as Poster Presentation by the 4th Biennial Congress of the Asian-Pacific Hepato-Pancreato-Biliary Association (A-PHPBA) 2013.
Author’s contributions: Valeriy V. Boyko, designed study, analyzed data; Denys I. Skoryi, designed study, performed the experiment, developed methods, analyzed data; Margarita E. Pisetska, designed study, performed the experiment; Oleksandr M. Tyshchenko, analyzed data; Tatiana V. Kozlova, performed the experiment, drafted paper; Igor V. Volchenko, performed the experiment, drafted paper.
Funding: Public financing.
Disclosure: The authors declare no conflict of interest.
References
- Abu-Amara M, Gurusamy K, Hori S, et al. Systematic review of randomized controlled trials of pharmacological interventions to reduce ischaemia-reperfusion injury in elective liver resection with vascular occlusion. HPB (Oxford) 2010;12:4-14. [PubMed]
- Lau WY, Lai EC, Lau SH. Methods of vascular control technique during liver resection: a comprehensive review. Hepatobiliary Pancreat Dis Int 2010;9:473-81. [PubMed]
- Gurusamy KS, Sheth H, Kumar Y, et al. Methods of vascular occlusion for elective liver resections. Cochrane Database Syst Rev 2009;CD007632. [PubMed]
- Li SQ, Liang LJ, Huang JF, et al. Ischemic preconditioning protects liver from hepatectomy under hepatic inflow occlusion for hepatocellular carcinoma patients with cirrhosis. World J Gastroenterol 2004;10:2580-4. [PubMed]
- Tapuria N, Junnarkar S, Abu-Amara M, et al. Modulation of microcirculatory changes in the late phase of hepatic ischaemia-reperfusion injury by remote ischaemic preconditioning. HPB (Oxford) 2012;14:87-97. [PubMed]
- Clavien PA, Yadav S, Sindram D, et al. Protective effects of ischemic preconditioning for liver resection performed under inflow occlusion in humans. Ann Surg 2000;232:155-62. [PubMed]
- Smyrniotis V, Theodoraki K, Arkadopoulos N, et al. Ischemic preconditioning versus intermittent vascular occlusion in liver resections performed under selective vascular exclusion: a prospective randomized study. Am J Surg 2006;192:669-74. [PubMed]
- Yadav SS, Sindram D, Perry DK, et al. Ischemic preconditioning protects the mouse liver by inhibition of apoptosis through a caspase-dependent pathway. Hepatology 1999;30:1223-31. [PubMed]
- Li SQ, Liang LJ, Huang JF, et al. Ischemic preconditioning protects liver from hepatectomy under hepatic inflow occlusion for hepatocellular carcinoma patients with cirrhosis. World J Gastroenterol 2004;10:2580-4. [PubMed]