Are hematopoietic stem cells involved in hepatocarcinogenesis?
Introduction
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide and is a main cause of cancer-related death (1).
Stem cells have the highest potential for proliferation and possess a longer life span compared with their progeny (2,3). It has been suggested that stem cells have two unique properties that make them likely to be involved in cancer development (3). First, they are often in a tissue the only long-living cells that have the ability to replicate. Before a cell becomes cancerous, multiple mutations occurring over many years are necessary and it has been suggested that these long-living stem cells have the greatest opportunity to accumulate such cancer-inducing mutations while remaining viable (3). Second, through a process called self-renewal, stem cells generate new stem cells with similar proliferation and differentiation capacities. However, because the same self-renewal mechanism occurs in both normal and cancerous cells, it has been suggested that cancers arise either from normal stem cells or from progenitor cells in which self-renewal pathways have become deranged (3). In other words, cancer can be regarded as a disease of unregulated self-renewal (2-4).
Stem cells exist in the hematopoietic system and in several other tissues and the delineation of their properties and genetic programs are now matters of the regenerative medicine. It’s well known that tumors may originate from the transformation of normal stem cells, and also within cancer cells it is possible to recognize “cancer stem cells”—rare cells with indefinite potential for self-renewal—that drive tumorigenesis (5).
Stem cells and hepatic proliferation
Since only proliferating cells can trigger a carcinogenetic process, in the last years many studies have been published on the characteristics of such cells in the liver.
The liver has three cell lineages able to proliferate after a hepatic injury:
- The mature hepatocyte, which proliferates after partial hepatectomy (PH) or centrolobular injury, such as that induced by carbon tetrachloride (CCl4), and dimethylnitrosamine (DEN);
- The ductular “bipolar” progenitor cell, termed oval cell, which responds to centrolobular injury when the proliferation of hepatocytes is inhibited, such as in case of N-2-acetylaminofluorene (AAF) administration;
- The putative periductular stem cell, which responds to periportal injury, such as that induced by allyl alcohol or observed in choline-deficiency or (fumarylacetoacetate hydrolase deficent) Fah–/– mice models (see below in text).
Hepatocytes can only produce other hepatocytes whereas ductular progenitor cells are considerate bipolar since they can give rise to biliary cells or hepatocytes (6).
Periductular stem cells are rare in the liver, have a very long proliferation potential, and may be multipotent, being this aspect still under investigation. They originate in the bone marrow since their progeny express genetic markers of donor hematopoietic cells after bone marrow transplantation (7).
Thus, experimental models of liver injury and of hepatocarcinogenesis may call forth a cellular response at different levels in the hepatic lineage (heterogeneity), having these cells different potential to form cells of other types (plasticity) (6).
Since the liver is the hematopoietic organ of the fetus, it is possible that hematopoietic stem cells may reside in the liver of the adult. This assumption is proved by the finding that oval cells express hematopoietic markers like CD34, CD45, CD109, Thy-1, c-kit, and others, which are also expressed by bone marrow-derived hematopoietic stem cells (7). Hence, oval cells might be candidate progeny of hematopoietic stem cells in the liver, which has been shown in cross-sex or cross-strain bone marrow and whole liver transplantation experiments using a mouse model of 2-acetylaminofluorene/CCl4 intoxication (8). In such models the conversion of bone marrow-derived cells to oval cells has been observed but the functional repopulation capacity of the hepatocytes derived was rather low (8). In addition, the responsiveness of oval cell CXCR4 receptor to the stromal-derived factor 1a (SDF-1a) after a hepatic injury further supports the demonstration that oval cells descend from bone marrow-derived hematopoietic stem cells (9).
However, using three different models of oval cell activation in the rat, (D-galactosamine, retrorsine/partial hepatectomy, and 2-acetylaminofluorene/partial hepatectomy) no transdifferentiation of bone marrow-derived cells to oval cells was observed (9). Hence, in most of the animal models investigated till now, functional repopulation of the host liver by transplanted hepatocytes differentiated from hematopoietic stem cells is marginal. For instance, in the Fah–/– deficient mouse model, repopulation of a host liver by transplanted bone marrow stem cells (BMSCs) was nearly as efficient as with adult hepatocytes (10). Yet, hepatocytes were not transdifferentiated from BMSCs, but were a fusion product of host hepatocytes and marrow-derived cells (11,12). Recently, hematopoietic myelomonocytic cells have been identified as the major source of the host hepatocyte fusion partners (13,14).
In addition to hematopoietic stem cells, rodent and human bone marrow harbors CD34- and CD45-negative cells of mesenchymal origin, capable of multiple differentiation, which can be propagated in vitro and differentiate into cells with mesodermal or endodermal phenotype (15). These cells, termed mesenchymal stem cells (MSCs), if transplanted, lead to engraftment of different organs and differentiation into organ-specific cell types (16). Evidences suggest that a subpopulation of MSCs, the multipotent adult progenitor cells (MAPCs), could have hepatogenic and biliary differentiation potential, though in vivo hepatic tissue repopulation still awaits proof (17).
Studies in vitro have shown that human MSCs from bone marrow gained the characteristic morphology and selected functions of hepatocytes such as glycogen storage, urea synthesis, and activation of hepatocyte-specific gene promoters (18). A typical mesenchymal marker set is expressed in undifferentiated MSCs comprising CD13, CD29, CD44, CD54, CD90, CD105, and CD166 but lacking hematopoietic markers such as CD14, CD34, and CD45 (18). Through the hepatocytic differentiation in vitro, morphology from a fibroblastoid changed into a polygonal shape typical for hepatocytes and increased expression of functional hepatocyte markers like the periportal and perivenous marker enzymes phosphoenolpyruvate carboxykinase and glutamine synthase, respectively, as well as expression of the plasma protein alpha-1 antitrypsin (18).
Hence, there is increasing evidence that both hepatocytes and cholangiocytes are the final result of a progeny constituted by bipotent committed progenitor cells. It would be important to understand whether the heterogeneity of the ductular reactions appearing in the human liver after different kinds of injury (intoxication, viral infections or biliary obstructions) represents a differential answer of one type of stem cell to different challenges or the response of different types of stem cells or even different stem cell niches (19,20).
However, even if basic understanding of the cellular and molecular mechanisms of hepatocyte stem cell differentiation is emerging, it is still necessary to investigate the basic principles of hepatocyte regeneration deriving from stem cells both of hepatic and non-hepatic origin, in order to promote cellular integration and functional hepatic repair in therapeutic approaches.
Stem cells involved in liver physiopathology are listed in Table 1.

Full table
Stem cells and tumors
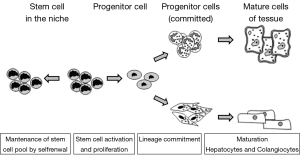
Stem cells are organized in niches where they are present in small numbers and in a quiescent state with a very slow rate of division (21,22). The division of stem cells produces one identical quiescent daughter cell and a transient amplifier cell that has a limited life span and is responsible for the subsequent cell divisions (22). The maintenance of a relatively inactive state is believed to depend on the stem cell niche and is necessary to protect their genome from mutations and avoid their overgrowth (Figure 1).
The concept that stem cells have a role in tumorigenesis is based on recent observations. Tumors are a heterogeneous mixture of tumor cells at various levels of differentiation, similar to the structure of an organ. This fits with the observation that most cancers comprise a heterogeneous population of cells that have undergone varying degrees of differentiation. In vivo and in vitro tumor studies have demonstrated that only a small fraction of cells within a tumor possess self-renewal capacity (23,24). This was proved, for the first time, by Dick et al. that demonstrated the presence of cells within the tumor with the capacity to reconstitute the tumor when transplanted into an appropriate recipient (differentiation) through several rounds of transplantation (self-renewal). The identification of tumor cell subpopulations with stem cell properties within breast tumors, gliomas, melanoma, prostate cancer and osteosarcoma confirms the “cancer stem cell (CSC) hypothesis” (25-31). Furthermore, conventional anti-cancer therapies targeting dividing cells may substantially reduce tumor bulk but rarely prevent tumor re-growth presumably because they are not able to destroy cancer stem cells.
Recent studies have identified promoters involved in self-renewal mechanism of CSCs such as Nanog, SOX2, Bmi. They might provide information on behavior of tumors, leading to a new strategy for targeting CSCs (32,33).
Hepatocarcinogenesis from stem cells
Recent evidences suggest the existence of liver cancer stem cells (LCSCs), but their origin is still unclear. There are two main hypotheses to explain the origin of LCSCs: the dedifferentiation of mature hepatocytes and the maturation arrest of liver stem cells. Early studies in rat models mainly focused on premalignant foci and nodules supported the dedifferentiation hypothesis (34,35).
However, this hypothesis has been challenged by subsequent researches. At present, it is commonly believed that liver stem/progenitor cells are the potential source of HCC, intrahepatic cholangiocarcinoma, combined hepatocellular cholangiocarcinoma and cholangiolocellular carcinoma (36-41).
In order to study the effect of oval cells upon tumorigenesis, de Lima et al. established a rat model of non-alcoholic steatohepatitis (NASH), cirrhosis and HCC, showing that oval cells could proliferate in this model and that these cells may be the origin of malignancy (42). In another model (the Solt-Farber carcinogenic model), hepatic progenitor cells, identified by the expression of glypican-3 (GPC3), were shown to play an important role in hepatic carcinogenesis (43).
It is observed that 55% of small cells in dysplastic foci, the earliest premalignant lesions in human HCC, consist of progenitor cells and intermediate hepatocytes (44).
In order to isolate LCSCs from HCC tissues, several biomarkers have been identified, including CD133, EpCAM and ABCG2 (45).
Among these markers, main role has been identified in CD 133, as shown in a severe PH model (45). Using this model, the mouse homologue of CD133 Prominin-1 was identified as the second most highly up-regulated gene after alphafetoprotein (AFP) in liver regeneration (45). CD133, a 5 transmembrane domain cell-surface glycoprotein, is regarded as an important marker for the identification and isolation of primitive stem/progenitor cells in both hematopoietic and non-hematopoietic tissues. It was originally found on hematopoietic stem cells and hematopoietic progenitor cells deriving from human fetal liver, bone marrow, and peripheral blood (46). Recently, it has been suggested that stem/progenitor cells expressing CD133 isolated from the bone marrow are able to repopulate up to 10% of the normal liver when transplanted (46).
Role of BMSCs in the hepatocarcinogenesis
The putative oncogenicity of BMSCs has been demonstrated by several studies: Murata et al. reported that BMSCs are a type of progenitor cell of malignant fibroma (47). Moreover, BMSCs may be the origin of Ewing tumor cells (48) and can be involved in breast cancer (49,50).
BMSCs seem to contribute to oncogenesis via two mechanisms: first, inducing angiogenesis by secreting pro-angiogenic factors and differentiating into endothelial-like or pericyto-like cells (51); second, in the tumor microenvironment they produce immunosuppressive factors to inhibit the proliferation of immunocytes and block the antigen-presenting process, thus allowing tumor cells to escape from immuno-surveillance (52).
BMSCs play a putative role in many aspects of tumor microenvironment. In the last years in fact, microenvironment surrounding HCC cells have been extensively studied as an important cofactor for tumor growth and aggressiveness (53).
Among the most investigated aspects of HCC microenvironment there are the control of angiogenesis (by means of VEGF expression), production of chemokines (such as CXCL12, CX3CL1, CCL20), regulation of Hypoxia induced factor-1 (HIF-1), a major transcription factor that regulates the expression of several genes with critical roles in angiogenesis, immune evasion, invasion and metastasis (53-55).
Moreover, other aspect are under investigation: in fact, overproduction of ROS provokes nitrosative and oxidative stress through interaction with DNA, RNA, lipid and proteins, leading to an increase in mutations, genomic instability, epigenetic changes, and protein dysfunction (53). Autophagy, a catabolic process up-regulated under metabolic stress conditions, is induced in tumor microenvironment. Stromal components are exposed to oxidative stress conditions induced by cancer cells that together with hypoxia induce autophagy (56). Autophagy in the tumor stroma acts as a pro-survival mechanism that generates energy able to fuel cancer cells alleviating the metabolic imbalance and promoting their survival (53-56).
On the other hand, a number of studies have demonstrated a non-univocal role of BMSCs in several tumoral diseases. Cogle et al. showed that BMSCs do not contribute to skin cancer (57). In particular, they studied epithelial neoplasias found in patients after hematopoietic cell transplantation and demonstrated that human marrow incorporates into neoplasias by adopting the phenotype of the surrounding neoplastic environment, a process known as “developmental mimicry” (57).
Few studies have evaluated the role of BMSC in hepatocarcinogenesis so far. Kubota et al. have shown that bone marrow-derived cells fused with hepatic oval cells are not involved in hepatic tumorigenesis in the choline-deficient ethionine (CDE) supplemented diet rat model (8). This type of diet induces preneoplastic nodules and hepatocellular carcinoma derived from oval cell progenitors; they have concluded that fused oval cells and BMCs might not have malignant potential in the CDE-treated rat model (8).
Moreover, Ishikawa et al. using a chemically induced cancer model in rodents, did not find any participation of BMSCs in hepatocarcinogenesis (58).
Several injured liver models have shown that the inflammatory mediators (chemokines, cytokines, and adhesion molecules) participate to the mobilization of BMSCs and their migration and homing into the liver (59-61). Due to the well-known carcinogenetic role of inflammation (62,63), a common pathway between the two factors (BMSCs and inflammation) can be argued and such a finding has been confirmed by recent studies conducted in chemically or genetically induced HCCs (64-66).
In a recent study we evaluated the possibility that bone marrow-derived stem cells could participate to the neoplastic growth in the hepatitis B virus (HBV) transgenic mouse model of hepatocarcinogenesis described by Chisari et al. (67). In this model hepatocytes develop progressive damage and inflammation, due to intracellular accumulation of HBsAg protein (68). Such condition induces a compensatory proliferative reaction that involves not only hepatocytes but also small basophilic hepatocytes, bile duct cells, and oval cells. This complex of alterations leads to the development of dysplastic lesions, which progresses to liver cancer (68).
In our experiment, female HBV transgenic mice were sublethally irradiated and transplanted with bone marrow cells obtained from age-matched, wild type male mice. The fate of bone marrow cells was followed through the detection of the Y-chromosome. Finally, to characterize the cells within the liver tissues was used HBs-Pr and HBsAg encoding gene (HBs-Eg) which accumulates respectively into the cytoplasm and in the nucleus of “normal” female hepatocytes but not in neoplastic cells, and the hepatocyte lineage marker hepatocyte nuclear factor 1 (HNF1) (67). Finally, Y-chromosome-positive cells were always found in “normal” or regenerative areas and were never localized in neoplastic foci/nodules (HBs-Pr/HBs-Eg-negative). Simultaneous detection of both Y-chromosome and HBs-Eg in the nucleus of an HNF1-positive cell (hepatocyte) demonstrates a phenomenon of cell fusion. In conclusion our experiment demonstrates that BMSCs participate in the hepatic regenerative process but not in neoplastic growth (67).
Conclusions
The oncogenetic role of BMSCs in inducing HCC has not been completely explained yet. In particular, several controversies on their direct participation in hepatocarcinogenesis remain. Further studies in vitro and in vivo are warranted in order to definitively clarify such an issue.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- El-Serag HB. Hepatocellular carcinoma. N Engl J Med 2011;365:1118-27. [PubMed]
- Lee AS, Tang C, Rao MS, et al. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat Med 2013;19:998-1004. [PubMed]
- Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature 2001;414:105-11. [PubMed]
- Diehl AM. Neighborhood watch orchestrates liver regeneration. Nat Med 2012;18:497-9. [PubMed]
- Li L, Neaves WB. Normal stem cells and cancer stem cells: the niche matters. Cancer Res 2006;66:4553-7. [PubMed]
- Sell S. Heterogeneity and plasticity of hepatocyte lineage cells. Hepatology 2001;33:738-50. [PubMed]
- Li J, Xin J, Zhang L, et al. Human hepatic progenitor cells express hematopoietic cell markers CD45 and CD109. Int J Med Sci 2013;11:65-79. [PubMed]
- Kubota K, Soeda J, Misawa R, et al. Bone marrow-derived cells fuse with hepatic oval cells but are not involved in hepatic tumorigenesis in the choline-deficient ethionine-supplemented diet rat model. Carcinogenesis 2008;29:448-54. [PubMed]
- Menthena A, Deb N, Oertel M, et al. Bone marrow progenitors are not the source of expanding oval cells in injured liver. Stem Cells 2004;22:1049-61. [PubMed]
- Pilat N, Unger L, Berlakovich GA. Implication for Bone Marrow Derived Stem Cells in Hepatocyte Regeneration after Orthotopic Liver Transplantation. Int J Hepatol 2013;2013:310612.
- Almeida-Porada G, Zanjani ED, Porada CD. Bone marrow stem cells and liver regeneration. Exp Hematol 2010;38:574-80. [PubMed]
- Vassilopoulos G, Wang PR, Russell DW. Transplanted bone marrow regenerates liver by cell fusion. Nature 2003;422:901-4. [PubMed]
- Willenbring H, Bailey AS, Foster M, et al. Myelomonocytic cells are sufficient for therapeutic cell fusion in liver. Nat Med 2004;10:744-8. [PubMed]
- Camargo FD, Finegold M, Goodell MA. Hematopoietic myelomonocytic cells are the major source of hepatocyte fusion partners. J Clin Invest 2004;113:1266-70. [PubMed]
- Wakao S, Kuroda Y, Ogura F, et al. Regenerative Effects of Mesenchymal Stem Cells: Contribution of Muse Cells, a Novel Pluripotent Stem Cell Type that Resides in Mesenchymal Cells. Cells 2012;1:1045-60. [PubMed]
- Sousa BR, Parreira RC, Fonseca EA, et al. Human adult stem cells from diverse origins: an overview from multiparametric immunophenotyping to clinical applications. Cytometry A 2014;85:43-77. [PubMed]
- Schwartz RE, Reyes M, Koodie L, et al. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest 2002;109:1291-302. [PubMed]
- Christ B, Pelz S. Implication of hepatic stem cells in functional liver repopulation. Cytometry A 2013;83:90-102. [PubMed]
- Machado MV, Diehl AM. Liver renewal: detecting misrepair and optimizing regeneration. Mayo Clin Proc 2014;89:120-30. [PubMed]
- Li L, Neaves WB. Normal stem cells and cancer stem cells: the niche matters. Cancer Res 2006;66:4553-7. [PubMed]
- Scadden DT. Nice neighborhood: emerging concepts of the stem cell niche. Cell 2014;157:41-50. [PubMed]
- Yamashita YM, Tumbar T. Stem cells and their niche in homeostasis/regeneration and disease. Mol Biol Cell 2014;25:736. [PubMed]
- Ito K, Suda T. Metabolic requirements for the maintenance of self-renewing stem cells. Nat Rev Mol Cell Biol. 2014;15:243-56. [PubMed]
- Harrington L. Does the reservoir for self-renewal stem from the ends? Oncogene 2004;23:7283-9. [PubMed]
- Al-Hajj M, Wicha MS, Benito-Hernandez A, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A 2003;100:3983-8. [PubMed]
- Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res 2003;63:5821-8. [PubMed]
- Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature 2004;432:396-401. [PubMed]
- Sanai N, Alvarez-Buylla A, Berger MS. Neural stem cells and the origin of gliomas. N Engl J Med 2005;353:811-22. [PubMed]
- Fang D, Nguyen TK, Leishear K, et al. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res 2005;65:9328-37. [PubMed]
- Collins AT, Berry PA, Hyde C, et al. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res 2005;65:10946-51. [PubMed]
- Gibbs CP, Kukekov VG, Reith JD, et al. Stem-like cells in bone sarcomas: implications for tumorigenesis. Neoplasia 2005;7:967-76. [PubMed]
- Gong Y, Guo Y, Hai Y, et al. Nodal promotes the self-renewal of human colon cancer stem cells via an autocrine manner through Smad2/3 signaling pathway. Biomed Res Int 2014;2014:364134.
- Amini S, Fathi F, Mobalegi J, et al. The expressions of stem cell markers: Oct4, Nanog, Sox2, nucleostemin, Bmi, Zfx, Tcl1, Tbx3, Dppa4, and Esrrb in bladder, colon, and prostate cancer, and certain cancer cell lines. Anat Cell Biol 2014;47:1-11. [PubMed]
- Gournay J, Auvigne I, Pichard V, et al. In vivo cell lineage analysis during chemical hepatocarcinogenesis in rats using retroviral-mediated gene transfer: evidence for dedifferentiation of mature hepatocytes. Lab Invest 2002;82:781-8. [PubMed]
- Bralet MP, Pichard V, Ferry N. Demonstration of direct lineage between hepatocytes and hepatocellular carcinoma in diethylnitrosamine-treated rats. Hepatology 2002;36:623-30. [PubMed]
- Dumble ML, Croager EJ, Yeoh GC, et al. Generation and characterization of p53 null transformed hepatic progenitor cells: oval cells give rise to hepatocellular carcinoma. Carcinogenesis 2002;23:435-45. [PubMed]
- Fujii T, Zen Y, Harada K, et al. Participation of liver cancer stem/progenitor cells in tumorigenesis of scirrhous hepatocellular carcinoma--human and cell culture study. Hum Pathol 2008;39:1185-96. [PubMed]
- Nomoto K, Tsuneyama K, Cheng C, et al. Intrahepatic cholangiocarcinoma arising in cirrhotic liver frequently expressed p63-positive basal/stem-cell phenotype. Pathol Res Pract 2006;202:71-6. [PubMed]
- Tanaka S, Yamamoto T, Tanaka H, et al. Potentiality of combined hepatocellular and intrahepatic cholangiocellular carcinoma originating from a hepatic precursor cell: Immunohistochemical evidence. Hepatol Res 2005;32:52-7. [PubMed]
- Zhang F, Chen XP, Zhang W, et al. Combined hepatocellular cholangiocarcinoma originating from hepatic progenitor cells: immunohistochemical and double-fluorescence immunostaining evidence. Histopathology 2008;52:224-32. [PubMed]
- Komuta M, Spee B, Vander Borght S, et al. Clinicopathological study on cholangiolocellular carcinoma suggesting hepatic progenitor cell origin. Hepatology 2008;47:1544-56. [PubMed]
- de Lima VM, Oliveira CP, Alves VA, et al. A rodent model of NASH with cirrhosis, oval cell proliferation and hepatocellular carcinoma. J Hepatol 2008;49:1055-61. [PubMed]
- Grozdanov PN, Yovchev MI, Dabeva MD. The oncofetal protein glypican-3 is a novel marker of hepatic progenitor/oval cells. Lab Invest 2006;86:1272-84. [PubMed]
- Ma S, Chan KW, Hu L, et al. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology 2007;132:2542-56. [PubMed]
- Gehling UM, Willems M, Dandri M, et al. Partial hepatectomy induces mobilization of a unique population of haematopoietic progenitor cells in human healthy liver donors. J Hepatol 2005;43:845-53. [PubMed]
- am Esch JS 2nd, Knoefel WT, Klein M, et al. Portal application of autologous CD133+ bone marrow cells to the liver: a novel concept to support hepatic regeneration. Stem Cells 2005;23:463-70.
- Murata M, Matsuzaki K, Yoshida K, et al. Hepatitis B virus X protein shifts human hepatic transforming growth factor (TGF)-beta signaling from tumor suppression to oncogenesis in early chronic hepatitis B. Hepatology 2009;49:1203-17. [PubMed]
- Tirode F, Laud-Duval K, Prieur A, et al. Mesenchymal stem cell features of Ewing tumors. Cancer Cell 2007;11:421-9. [PubMed]
- Mishra PJ, Mishra PJ, Humeniuk R, et al. Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res 2008;68:4331-9. [PubMed]
- Avital I, Moreira AL, Klimstra DS, et al. Donor-derived human bone marrow cells contribute to solid organ cancers developing after bone marrow transplantation. Stem Cells 2007;25:2903-9. [PubMed]
- Prigione I, Benvenuto F, Bocca P, et al. Reciprocal interactions between human mesenchymal stem cells and gammadelta T cells or invariant natural killer T cells. Stem Cells 2009;27:693-702. [PubMed]
- Galiè M, Konstantinidou G, Peroni D, et al. Mesenchymal stem cells share molecular signature with mesenchymal tumor cells and favor early tumor growth in syngeneic mice. Oncogene 2008;27:2542-51. [PubMed]
- Hernandez-Gea V, Toffanin S, Friedman SL, et al. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology 2013;144:512-27. [PubMed]
- Roussos ET, Condeelis JS, Patsialou A. Chemotaxis in cancer. Nat Rev Cancer 2011;11:573-87. [PubMed]
- Semenza GL. Oxygen sensing, homeostasis, and disease. N Engl J Med 2011;365:537-47. [PubMed]
- Lisanti MP, Martinez-Outschoorn UE, Chiavarina B, et al. Understanding the “lethal” drivers of tumor-stroma co-evolution: emerging role(s) for hypoxia, oxidative stress and autophagy/mitophagy in the tumor micro-environment. Cancer Biol Ther 2010;10:537-42. [PubMed]
- Cogle CR, Theise ND, Fu D, et al. Bone marrow contributes to epithelial cancers in mice and humans as developmental mimicry. Stem Cells 2007;25:1881-7. [PubMed]
- Ishikawa H, Nakao K, Matsumoto K, et al. Bone marrow engraftment in a rodent model of chemical carcinogenesis but no role in the histogenesis of hepatocellular carcinoma. Gut 2004;53:884-9. [PubMed]
- Houghton J, Stoicov C, Nomura S, et al. Gastric cancer originating from bone marrow-derived cells. Science 2004;306:1568-71. [PubMed]
- Dalakas E, Newsome PN, Harrison DJ, et al. Hematopoietic stem cell trafficking in liver injury. FASEB J 2005;19:1225-31. [PubMed]
- Piscaglia AC, Shupe TD, Oh SH, et al. Granulocyte-colony stimulating factor promotes liver repair and induces oval cell migration and proliferation in rats. Gastroenterology 2007;133:619-31. [PubMed]
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001;357:539-45. [PubMed]
- Mantovani A, Balkwill F. RalB signaling: a bridge between inflammation and cancer. Cell 2006;127:42-4. [PubMed]
- Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol 2005;5:749-59. [PubMed]
- Pikarsky E, Porat RM, Stein I, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature 2004;431:461-6. [PubMed]
- Sakurai T, Maeda S, Chang L, et al. Loss of hepatic NF-kappa B activity enhances chemical hepatocarcinogenesis through sustained c-Jun N-terminal kinase 1 activation. Proc Natl Acad Sci U S A 2006;103:10544-51. [PubMed]
- Barone M, Scavo MP, Maiorano E, et al. Bone marrow-derived stem cells and hepatocarcinogenesis in hepatitis B virus transgenic mice. Dig Liver Dis 2014;46:243-50. [PubMed]
- Chisari FV, Pinkert CA, Milich DR, et al. A transgenic mouse model of the chronic hepatitis B surface antigen carrier state. Science 1985;230:1157-60. [PubMed]