Prolonged administration of secretin to normal rats increases biliary proliferation and secretin-induced ductal secretory activity
Introduction
Intrahepatic bile duct epithelial cells (i.e., cholangiocytes), which line the biliary ductal system, modify cholangiocyte bile into its final composition before reaching the small intestine (1-4). Ductal bile secretion is coordinately regulated by several gastrointestinal peptides/hormones including gastrin, endothelin-1, somatostatin and secretin that display inhibitory and stimulatory effects on bile and bicarbonate secretion (2,4-7). Among these factors, secretin stimulates water and bicarbonate secretion by interaction with basolateral secretin receptors (SR) that are expressed in the liver only by large bile ducts (1,8-11). The specific interaction of secretin with SR induces an increase in cyclic adenosine 3',5'-monophosphate (cAMP) levels (1,8,9,12,13), opening of the Cl– channel [cystic fibrosis transmembrane conductance regulator (CFTR)] (14), which leads to the activation of the Cl–/HCO3– anion exchanger 2 (15) and bicarbonate ductal secretion (2,6).
In addition to modifying ductal bile (2,4-7), cholangiocytes (normal mitotically dormant) (16,17) are the capacity to markedly proliferate or undergo apoptosis in response to pathological maneuvers such as extrahepatic bile duct ligation (BDL), partial hepatectomy, acute carbon tetrachloride (CCl4) or chronic administration of gamma-aminobutyric acid (16-19). While some of these maneuvers induce biliary proliferation (e.g., BDL and partial hepatectomy) (2,16,17), others cause bile duct damage (18,19). A number of studies have shown that enhanced biliary proliferation is associated with increased SR expression and secretin-stimulated ductal secretory activity (2,11,14,17). Conversely, damage of bile ducts is associated with reduced secretin-stimulated ductal secretion (18,19). The proliferative response of large cholangiocytes (the only liver cells expressing SR) (1,8) is regulated by activation of the cAMP-dependent PKA/ERK1/2 signaling (16,20,21). A previous study suggests that activation of the secretin SR axis stimulates cholangiocyte growth in mice, since knockout of the SR gene inhibits hyperplasia in cholestatic mice (22); however, no data exists regarding the trophic effect of secretin on normal rats. Thus, we performed studies in normal rats aimed to determine if secretin stimulates biliary proliferation and secretin-stimulated ductal secretory activity (functional indices of biliary proliferation) (1,2,16) by a cAMP-dependent signaling.
Methods and materials
Materials
Reagents were purchased from Sigma Chemical Co. (St. Louis, MO, USA) unless otherwise indicated. Porcine secretin was purchased from Peninsula Laboratories (Belmont, CA, USA). The substrate for γ-glutamyltranspeptidase (γ-GT), N (γ-L-glutamyl)-4-methoxy-2-naphthylamide, was purchased from Polysciences (Warrington, PA, UK). The mouse anti-cytokeratin-19 (CK-19) antibody was purchased from Caltag Laboratories Inc. (Burlingame, CA, USA). The EIA kits for the measurement of intracellular cAMP levels in cholangiocytes were obtained from Cayman Chemical (Ann Arbor, MI, USA).
Animal models
Male Fisher rats (150-175 gm) were purchased from Charles River (Wilmington, MA, USA). The animals were maintained in a temperature-controlled environment (20-22 °C) with 12: 12-hour light-dark cycles. All animal experiments were performed in accordance with protocols approved by the Scott and White and Texas A&M Health Science Center College of Medicine Institutional Animal Care and Use Committee. We used normal male treated with saline (0.9% NaCl) or secretin (3.12 nmol/kg body weight per day) (22,23) by intraperitoneally implanted Alzet® osmotic minipumps (Alzet, Cupertino, CA, USA) for seven days.
Freshly isolated cholangiocytes and normal intrahepatic cell lines
The in vitro studies were performed in freshly isolated cholangiocytes (6,24) and normal rat intrahepatic cholangiocyte lines (NRIC) (25). Virtually pure (by γ-GT histochemistry) (26) cholangiocytes were purified by immunoaffinity separation (6,24) by a monoclonal antibody, rat IgG2a (provided by Dr. R. Faris, Brown University, Providence, RI, USA), against an antigen expressed by all rat cholangiocytes (24). NRIC, which display phenotypic and functional phenotypes similar to that of freshly isolated cholangiocytes, were cultured as described (25).
Evaluation of cholangiocyte proliferation and intrahepatic bile duct mass (IBDM)
In liver sections (4-5 μm thick) from the two groups of animals, we measured IBDM (9) of cholangiocytes. The IBDM was measured as area occupied by CK-19 positive-bile ducts/total area ×100. Proliferation was also measured by real-time PCR in total RNA (1 μg) from purified cholangiocytes. Blots were normalized by β-actin (9,27). RNA was extracted from cholangiocytes by the RNeasy Mini Kit (Qiagen Inc, Valencia, CA, USA), and reverse transcribed using the Reaction Ready™ First Strand cDNA synthesis kit (SuperArray, Frederick, MD, USA). These reactions were used as templates for the PCR assays using a SYBR Green PCR master mix and specific primers designed against the rat PCNA (NM_022381), and the rat glyceraldehyde-3-phosphate dehydrogenase (GAPDH, NM_008084), the housekeeping gene (27) (SuperArray, Frederick, MD, USA) in the real-time thermal cycler (ABI Prism 7900HT sequence detection system). A ∆∆CT (delta delta of the threshold cycle) analysis was performed using normal cholangiocytes as the control sample. Data are expressed as relative mRNA levels ± SEM (n=3).
Measurement of mRNA expression of SR and CFTR and basal and secretin-stimulated cAMP Levels and bile and bicarbonate secretion
The mRNA expression of SR and CFTR, in purified cholangiocytes was evaluated by real-time PCR (27). The primers for rat SR and CFTR (SABiosciences) were designed according to the NCBI GenBank Accession numbers: NM_031115 (SR); XM_001059206 (CFTR); and GAPDH, NM_008084. A ∆∆CT analysis was performed using normal cholangiocytes as the control sample. Data are expressed as relative mRNA levels ± SEM (n=3).
Basal and secretin-stimulated cAMP levels (a functional parameter of cholangiocyte growth) (17,27,28) were measured in purified cholangiocytes (after incubation for one hour at 37 °C to regenerate SR potentially damaged by enzyme digestion during cell isolation) (12) by commercially available EIA kits (3,29,30).
In the in vivo studies of biliary physiology, after anesthesia (50 mg/kg BW, IP) rats were surgically prepared for bile collection as described by us (2). After steady-state bile flow was reached (60-70 min from the intravenous infusion of Krebs-Ringer-Henseleit solution, KRH), the animals were infused with secretin (100 nM for 30 minutes) via a jugular vein followed by intravenous infusion of KRH for 30 min (2). Bicarbonate levels in bile were determined by a COBAS Mira Plus automated clinical chemistry analyzer (Bohemia, NY, USA).
In vitro effect of secretin on the proliferation of NRIC
NRIC were treated at 37 °C with: 0.2% BSA (basal) or secretin (100 nM) for 24 to 72 hours in the absence or presence of pre-incubation (one hour) with H89 (a PKA inhibitor, 30 μM) (31) or PD98059 (a MEK inhibitor, 10 nM) (30) before evaluating proliferation by CellTiter 96 AQueous One Solution Cell Proliferation Assay (27) (Promega Corp., Madison, WI, USA). Absorbance was measured at 490 nm on a microplate spectrophotometer (Versamax, Molecular Devices, Sunnyvale, CA, USA).
Statistical analysis
All data are expressed as mean ± SEM. Differences between groups were analyzed by the Student’s unpaired t-test when two groups were analyzed, and by ANOVA when more than two groups were analyzed, followed by an appropriate post hoc test.
Results
Evaluation of cholangiocyte proliferation and IBDM
The prolonged administration of secretin to normal rats increased IBDM compared to saline-treated normal rats (Figure 1). In purified cholangiocytes from secretin-treated normal rats there was an increase in mRNA PCNA expression compared to large cholangiocytes from saline-treated rats (Figure 2A).

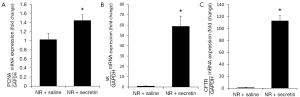
Measurement of mRNA expression of SR and CFTR and basal and secretin-stimulated cAMP levels and bile and bicarbonate secretion
As shown in Figure 2B,C, there was enhanced mRNA expression of SR and CFTR in purified cholangiocytes compared to normal rats treated with saline.
Secretin increased cAMP levels at higher levels in cholangiocytes from rats treated with secretin compared to saline-treated rats (Figure 3). In agreement with previous studies (2,6), intravenous infusion of secretin did not increase significantly bile and bicarbonate secretion of rats treated with saline for one week (Table 1). When administered to rats treated with secretin for one week, secretin enhanced significantly bile and bicarbonate secretion compared to basal values (Table 1).
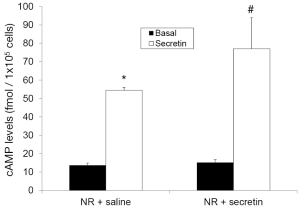
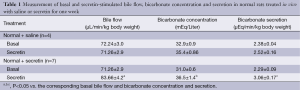
Full table
In vitro effect of secretin on the proliferation of NRIC
NRIC proliferate after the administration of secretin for 6-24 hr. Secretin-stimulation of NRIC proliferation was blocked by H89 and PD98059 (Figure 4). The data suggests that secretin stimulates in vitro large cholangiocyte proliferation by PKA/ERK1/2-dependent mechanism.
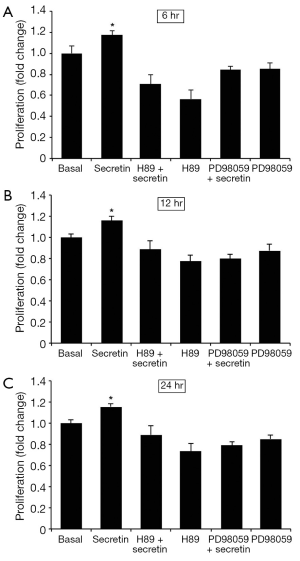
Discussion
In the present study, we demonstrated that: (I) secretin increases the proliferation of cholangiocytes from normal rats; and (II) secretin-induced increase in biliary proliferation is associated with enhanced expression of SR and CFTR and secretin-stimulated cAMP levels and bicarbonate-rich choleresis, functional indices of biliary proliferation (2,8,11,14). Specifically, we treated normal rats with secretin for one week and demonstrated an increase in IBDM in liver sections and PCNA expression in purified cholangiocytes compared to saline-treated normal rats. We also demonstrated that prolonged administration of secretin increased the expression of proteins (SR and CFTR) that are key in the regulating ductal secretory activity (1,2,8,16) and enhanced secretin-stimulated cAMP levels and bile and bicarbonate secretion. In in vitro studies, we demonstrated that secretin increased the proliferation of NRIC, increase that was prevented by PKA and MAPK inhibitors.
There is growing information regarding the role of gastrointestinal hormones/peptides in the regulation of biliary proliferation/damage (1,5,16,28,32,33). For example, studies have demonstrated that gastrin (by interaction with CCK-B/gastrin receptors) inhibits biliary hyperplasia in cholestatic rats by downregulation of Ca2+-dependent PKC isoforms (28,33). Also, the gastrointestinal hormone, somatostatin, has been shown to inhibit biliary proliferation by interaction with SSTR2 receptor (the only receptor subtype expressed by cholangiocytes) (5) by decreased expression of cAMP signaling (16). Prolonged administration of serotonin 1A and 1B receptors has also been shown to inhibit bile duct hyperplasia of cholestatic rats (32). Prolonged administration of gamma-aminobutyric acid induces the damage of large cholangiocytes and the de novo proliferation of small cholangiocytes and their differentiation into large cholangiocytes (19). Glucagon-like peptide-1 and Its receptor agonist, exendin-4, stimulates biliary proliferation of both normal and cholestatic rodents (34). While H3 histamine receptor agonists inhibit biliary proliferation of cholestatic rats (20), H1 histamine receptor agonists increase the proliferation of small cholangiocytes (27). Furthermore, melatonin has been shown to inhibit cholangiocyte proliferation in cholestatic rats by interaction with specific interaction with MT1 melatonin receptors (35). Furthermore, we have shown that follicle-stimulating hormone increases biliary growth by cAMP-dependent phosphorylation of ERK1/2 and Elk-1 (36). Our findings provide new evidence that secretin stimulates biliary proliferation of normal rats, a phenomenon that was associated with increased expression of secretin-induced signaling and enhanced responsiveness to secretin. Although a number of studies suggest that the increased ductal secretory may be due to enhanced cholangiocytes proliferation (2,11,14,17), our findings introduce the novel concept that prolonged administration of secretin may increase the expression of SR and CFTR and cAMP levels and choleretic effect in response to secretin, effects that may be independent from the enhanced proliferation of cholangiocytes. Since SR are only expressed by large cholangiocytes (1,8) and cAMP signaling is restricted to large cholangiocytes (1,8,30), we propose that secretin mainly target large cholangiocytes as demonstrated by enhanced large IBDM. In support of this concept, we have shown that secretin increased the proliferation of NRIC, increase that was prevented by PKA and MAPK inhibitors. Indeed, the cAMP-dependent signaling is an important regulator for the proliferation of large cholangiocytes (1,6,8,12,14,17). This also supported by a previous study showing that activation of adenylyl cyclase activity stimulates normal cholangiocyte proliferation by activation of the PKA/Src/MEK/ERK1/2 pathway (30). Furthermore, maintenance of cAMP levels prevents the functional damage of bile ducts induced by vagotomy (37).
In contrast to the stimulatory effect of secretin on biliary proliferation observed in the present and other studies (22), we have demonstrated that secretin inhibits cholangiocarcinoma growth (38). The different effect of secretin on the growth of cholangiocarcinoma may be due dysregulation in the coupling of the SR to Gαs and loss of cAMP response that is normally present for SR that when is stimulated triggers the activation of adenylate cyclase resulting in increased intracellular cAMP levels (38). This concept is supported by the fact that secretin stimulates the proliferation of normal human cell lines (38).
In summary, we have demonstrated that prolonged administration of secretin to normal rats induces an increase in biliary proliferation and IBDM and enhanced expression of SR and CFTR and increased choleretic response to secretin. The study has potential clinical relevance since secretin may be important for the management of ductopenic liver diseases.
Acknowledgements
This work was supported by the Dr. Nicholas C. Hightower Centennial Chair of Gastroenterology from Scott & White.
Disclosure: The authors declare no conflict of interest. This material is the result of work supported with resources and the use of facilities at the Central Texas Veterans Health Care System, Temple, Texas. The views presented are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
References
- Alpini G, Glaser S, Robertson W, et al. Large but not small intrahepatic bile ducts are involved in secretin-regulated ductal bile secretion. Am J Physiol 1997;272:G1064-74. [PubMed]
- Alpini G, Lenzi R, Sarkozi L, et al. Biliary physiology in rats with bile ductular cell hyperplasia. Evidence for a secretory function of proliferated bile ductules. J Clin Invest 1988;81:569-78. [PubMed]
- Francis H, LeSage G, DeMorrow S, et al. The alpha2-adrenergic receptor agonist UK 14,304 inhibits secretin-stimulated ductal secretion by downregulation of the cAMP system in bile duct-ligated rats. Am J Physiol Cell Physiol 2007;293:C1252-62. [PubMed]
- Kanno N, LeSage G, Glaser S, et al. Regulation of cholangiocyte bicarbonate secretion. Am J Physiol Gastrointest Liver Physiol 2001;281:G612-25. [PubMed]
- Tietz PS, Alpini G, Pham LD, et al. Somatostatin inhibits secretin-induced ductal hypercholeresis and exocytosis by cholangiocytes. Am J Physiol 1995;269:G110-8. [PubMed]
- Glaser SS, Rodgers RE, Phinizy JL, et al. Gastrin inhibits secretin-induced ductal secretion by interaction with specific receptors on rat cholangiocytes. Am J Physiol 1997;273:G1061-70. [PubMed]
- Caligiuri A, Glaser S, Rodgers RE, et al. Endothelin-1 inhibits secretin-stimulated ductal secretion by interacting with ETA receptors on large cholangiocytes. Am J Physiol 1998;275:G835-46. [PubMed]
- Alpini G, Roberts S, Kuntz SM, et al. Morphological, molecular, and functional heterogeneity of cholangiocytes from normal rat liver. Gastroenterology 1996;110:1636-43. [PubMed]
- Glaser SS, Gaudio E, Rao A, et al. Morphological and functional heterogeneity of the mouse intrahepatic biliary epithelium. Lab Invest 2009;89:456-69. [PubMed]
- Martínez-Ansó E, Castillo JE, Díez J, et al. Immunohistochemical detection of chloride/bicarbonate anion exchangers in human liver. Hepatology 1994;19:1400-6. [PubMed]
- Alpini G, Ulrich CD 2nd, Phillips JO, et al. Upregulation of secretin receptor gene expression in rat cholangiocytes after bile duct ligation. Am J Physiol 1994;266:G922-8. [PubMed]
- Kato A, Gores GJ, LaRusso NF. Secretin stimulates exocytosis in isolated bile duct epithelial cells by a cyclic AMP-mediated mechanism. J Biol Chem 1992;267:15523-9. [PubMed]
- Ueno Y, Alpini G, Yahagi K, et al. Evaluation of differential gene expression by microarray analysis in small and large cholangiocytes isolated from normal mice. Liver Int 2003;23:449-59. [PubMed]
- Alpini G, Ulrich C, Roberts S, et al. Molecular and functional heterogeneity of cholangiocytes from rat liver after bile duct ligation. Am J Physiol 1997;272:G289-97. [PubMed]
- Banales JM, Arenas F, Rodríguez-Ortigosa CM, et al. Bicarbonate-rich choleresis induced by secretin in normal rat is taurocholate-dependent and involves AE2 anion exchanger. Hepatology 2006;43:266-75. [PubMed]
- Alpini G, Glaser SS, Ueno Y, et al. Heterogeneity of the proliferative capacity of rat cholangiocytes after bile duct ligation. Am J Physiol 1998;274:G767-75. [PubMed]
- Lesage G, Glaser SS, Gubba S, et al. Regrowth of the rat biliary tree after 70% partial hepatectomy is coupled to increased secretin-induced ductal secretion. Gastroenterology 1996;111:1633-44. [PubMed]
- LeSage GD, Glaser SS, Marucci L, et al. Acute carbon tetrachloride feeding induces damage of large but not small cholangiocytes from BDL rat liver. Am J Physiol 1999;276:G1289-301. [PubMed]
- Mancinelli R, Franchitto A, Gaudio E, et al. After damage of large bile ducts by gamma-aminobutyric acid, small ducts replenish the biliary tree by amplification of calcium-dependent signaling and de novo acquisition of large cholangiocyte phenotypes. Am J Pathol 2010;176:1790-800. [PubMed]
- Francis H, Franchitto A, Ueno Y, et al. H3 histamine receptor agonist inhibits biliary growth of BDL rats by downregulation of the cAMP-dependent PKA/ERK1/2/ELK-1 pathway. Lab Invest 2007;87:473-87. [PubMed]
- Alvaro D, Onori P, Metalli VD, et al. Intracellular pathways mediating estrogen-induced cholangiocyte proliferation in the rat. Hepatology 2002;36:297-304. [PubMed]
- Glaser S, Lam IP, Franchitto A, et al. Knockout of secretin receptor reduces large cholangiocyte hyperplasia in mice with extrahepatic cholestasis induced by bile duct ligation. Hepatology 2010;52:204-14. [PubMed]
- Welch MG, Welch-Horan TB, Anwar M, et al. Brain effects of chronic IBD in areas abnormal in autism and treatment by single neuropeptides secretin and oxytocin. J Mol Neurosci 2005;25:259-74. [PubMed]
- Ishii M, Vroman B, LaRusso NF. Isolation and morphologic characterization of bile duct epithelial cells from normal rat liver. Gastroenterology 1989;97:1236-47. [PubMed]
- Alpini G, Phinizy JL, Glaser S, et al. Development and characterization of secretin-stimulated secretion of cultured rat cholangiocytes. Am J Physiol Gastrointest Liver Physiol 2003;284:G1066-73. [PubMed]
- Rutenburg AM, Kim H, Fischbein JW, et al. Histochemical and ultrastructural demonstration of gamma-glutamyl transpeptidase activity. J Histochem Cytochem 1969;17:517-26. [PubMed]
- Francis H, Glaser S, Demorrow S, et al. Small mouse cholangiocytes proliferate in response to H1 histamine receptor stimulation by activation of the IP3/CaMK I/CREB pathway. Am J Physiol Cell Physiol 2008;295:C499-513. [PubMed]
- Glaser S, Benedetti A, Marucci L, et al. Gastrin inhibits cholangiocyte growth in bile duct-ligated rats by interaction with cholecystokinin-B/Gastrin receptors via D-myo-inositol 1,4,5-triphosphate-, Ca(2+)-, and protein kinase C alpha-dependent mechanisms. Hepatology 2000;32:17-25. [PubMed]
- Glaser SS, Ueno Y, DeMorrow S, et al. Knockout of alpha-calcitonin gene-related peptide reduces cholangiocyte proliferation in bile duct ligated mice. Lab Invest 2007;87:914-26. [PubMed]
- Francis H, Glaser S, Ueno Y, et al. cAMP stimulates the secretory and proliferative capacity of the rat intrahepatic biliary epithelium through changes in the PKA/Src/MEK/ERK1/2 pathway. J Hepatol 2004;41:528-37. [PubMed]
- Alvaro D, Della Guardia P, Bini A, et al. Effect of glucagon on intracellular pH regulation in isolated rat hepatocyte couplets. J Clin Invest 1995;96:665-75. [PubMed]
- Marzioni M, Glaser S, Francis H, et al. Autocrine/paracrine regulation of the growth of the biliary tree by the neuroendocrine hormone serotonin. Gastroenterology 2005;128:121-37. [PubMed]
- Glaser S, Alvaro D, Ueno Y, et al. Gastrin reverses established cholangiocyte proliferation and enhanced secretin-stimulated ductal secretion of BDL rats by activation of apoptosis through increased expression of Ca2+- dependent PKC isoforms. Liver Int 2003;23:78-88. [PubMed]
- Marzioni M, Alpini G, Saccomanno S, et al. Glucagon-like peptide-1 and its receptor agonist exendin-4 modulate cholangiocyte adaptive response to cholestasis. Gastroenterology 2007;133:244-55. [PubMed]
- Renzi A, Glaser S, Demorrow S, et al. Melatonin inhibits cholangiocyte hyperplasia in cholestatic rats by interaction with MT1 but not MT2 melatonin receptors. Am J Physiol Gastrointest Liver Physiol 2011;301:G634-43. [PubMed]
- Mancinelli R, Onori P, Gaudio E, et al. Follicle-stimulating hormone increases cholangiocyte proliferation by an autocrine mechanism via cAMP-dependent phosphorylation of ERK1/2 and Elk-1. Am J Physiol Gastrointest Liver Physiol 2009;297:G11-26. [PubMed]
- LeSagE G, Alvaro D, Benedetti A, et al. Cholinergic system modulates growth, apoptosis, and secretion of cholangiocytes from bile duct-ligated rats. Gastroenterology 1999;117:191-9. [PubMed]
- Onori P, Wise C, Gaudio E, et al. Secretin inhibits cholangiocarcinoma growth via dysregulation of the cAMP-dependent signaling mechanisms of secretin receptor. Int J Cancer 2010;127:43-54. [PubMed]


