
A primary adenosquamous gallbladder carcinoma with sarcomatoid features
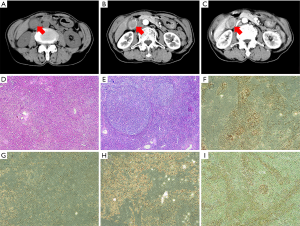
A 65-year-old female was admitted to our hospital complaining of abdominal pain for the past month. A physical examination revealed mild right upper quadrant tenderness without rebound pain and no palpable abdominal hepatomegaly or mass. Her laboratory results showed mildly impaired liver function: AST 51 U/L (normal range <34 U/L), ALT 49 U/L (normal range <34 U/L), and C-reactive protein 16.5 mg/L (normal range <10 mg/L). All other laboratory test results, including tumor markers, were within the normal range. The patient’s Child-Pugh score was grade A. A gallbladder mass was detected by contrast-enhanced CT and MRI of the abdomen and measured 32 mm× 23 mm. We observed heterogenous enhancement with central necrosis, as well as swollen lymph nodes among the hepatic hilar, common bile duct, and retroperitoneum. Imaging showed a gallbladder carcinoma with multiple lymph node metastases (Figure 1A,B,C). Radical gallbladder surgery was conducted to remove the entire gallbladder, part of liver segment V, and the lymph nodes. During the operation, an irregular hard mass was present at the base of the swollen gallbladder. Histologically, mixed cell types were identified. The adenosquamous carcinoma (ASC) region contained a diffuse, poorly differentiated adenocarcinoma with a centralized squamous cell carcinoma and the sarcomatoid carcinoma contained heterogeneous spindle cells with large nuclei and pleomorphic cells (Figure 1D,E). Immunohistochemistry (IHC) showed that the tumor cells were positive for CK [AE1/AE3, CK7, CK5/6, P63, P40, vimentin, and Ki-67 (60%)], and negative for p53, CgA, and Syn (Figure 1F,G,H,I). Following surgery, the patient was provided adjuvant chemotherapy with gemcitabine and oxaliplatin. At her 6-month follow-up exam, the patient had no symptoms and a good quality of life.
Adenocarcinomas are the most frequent malignant gallbladder neoplasm subtype, representing approximately 90–95% of all cases (1). However, the co-occurrence of adenocarcinoma and squamous cell carcinoma in the gallbladder is extremely rare. ASC is a malignant gallbladder tumor that contains at least 30% squamous cell carcinoma plus adenocarcinoma components. A sarcomatoid carcinoma includes malignant epithelial and mesenchymal cells. Kim et al. (2) reported an incidence of 2% for gallbladder ASC. Sarcomatoid carcinomas have only been documented in rare case reports. Cases of mixed adenosquamous and sarcomatoid carcinoma are exceedingly rare and have been shown in the thymus and esophagus (3). This is the first report of a gallbladder ASC with sarcomatoid changes.
In the present case, the neoplasm consisted of three histopathological types: adenocarcinoma, squamous carcinoma, and sarcoma. The pathological mechanism underlying this condition remains unclear given the limited number of reported cases. van den Berg et al. suggested that the three cell types share a monoclonal origin and subsequently diverge due to K-RAS mutations (4). Studies of hepato-sarcomatoid carcinomas have demonstrated that the tumor sarcoma cells are not mesenchymal and that anti-tumor treatments promote sarcomatoid changes (5). Lu et al. (6) used IHC to assess epithelial-mesenchymal transition (EMT) proteins; they discovered a loss of membranous E-cadherin expression and a gain of vimentin expression in tumor cells of the sarcomatoid lesion. However, strong E-cadherin expression and negative vimentin expression characterized the adenocarcinoma and squamous carcinoma portions of the lesion. It is possible that these three cell types originate from the same monoclonal origin and subsequently lose epithelial markers and acquire mesenchymal markers due to the effects of K-RAS mutations.
ASC presents as abdominal pain, making it difficult to differentiate from common gallbladder adenocarcinomas. Given their common features, imaging-based diagnoses are also challenging, but liver invasion and lymph node metastasis are more likely in ASC than gallbladder adenocarcinoma. Recognition of these unique features is important for the proper evaluation of local tumor invasion, distant metastasis, and clinical stage.
Ultimately, the diagnosis of ASC is achieved by the pathological evaluation of surgical or biopsy specimens. Radical surgery is recommended for gallbladder adenocarcinoma and ASC. Although patients receive similar surgical approaches, the prognosis for ASC is much worse than for adenocarcinoma. This may relate to the increased rate of ASC recurrence and metastasis. The effectiveness of chemoradiotherapy and radiotherapy does not outweigh that of neoplasm resection surgery. Thus, these therapies are typically used only in advanced gallbladder cases. Sarcomatoid changes are considered necessary for invasion and metastasis (7). In the present case, 60% of the sampled cells were positive for Ki-67, a proliferation marker used to estimate malignant potential and predict the risk of tumor recurrence. Consequently, the patient was treated with gemcitabine combined with oxaliplatin, a recommended adjuvant chemotherapy for advanced gallbladder cancer. Although sarcomatoid changes are associated with chemotherapy and radiotherapy resistance (8), cancer recurrence and metastases were not present at the 6-month follow-up evaluation and the patient reported a good quality of life. Since gallbladder ASC with sarcomatoid changes indicates a poor prognosis, we suggest aggressive treatment strategies for patients following radical surgery.
In summary, we present the first reported case of gallbladder ASC with sarcomatoid changes. The mixed cell types likely originated from a single cell, but additional studies are needed to clarify the underlying mechanism. It is currently not possible to precisely diagnose ASC by CT or MRI perioperatively given the shared features with common gallbladder carcinoma. We recommend aggressive treatment and radical surgery for this unusual malignant tumor to prolong survival and improve patient quality of life.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (No. 81572307 and 81773096).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Reid KM, Ramos-De la Medina A, Donohue JH. Diagnosis and surgical management of gallbladder cancer: a review. J Gastrointest Surg 2007;11:671-81. [Crossref] [PubMed]
- Kim WS, Jang KT, Choi DW, et al. Clinicopathologic analysis of adenosquamous/squamous cell carcinoma of the gallbladder. J Surg Oncol 2011;103:239-42. [Crossref] [PubMed]
- Liu YG, Sun KK, Sui XZ, et al. Thymic carcinosarcoma consisting of sarcomatous and adenosquamous carcinomatous component. Chin Med J (Engl) 2012;125:4154-5. [PubMed]
- van den Berg W, Tascilar M, Offerhaus GJ, et al. Pancreatic mucinous cystic neoplasms with sarcomatous stroma: molecular evidence for monoclonal origin with subsequent divergence of the epithelial and sarcomatous components. Mod Pathol 2000;13:86-91. [Crossref] [PubMed]
- Marijon H, Dokmak S, Paradis V, et al. Epithelial-to-mesenchymal transition and acquired resistance to sunitinib in a patient with hepatocellular carcinoma. J Hepatol 2011;54:1073-8. [Crossref] [PubMed]
- Lu BC, Wang C, Yu JH, et al. A huge adenosquamous carcinoma of the pancreas with sarcomatoid change: an unusual case report. World J Gastroenterol 2014;20:16381-6. [Crossref] [PubMed]
- Voulgari A, Pintzas A. Epithelial-mesenchymal transition in cancer metastasis: mechanisms, markers and strategies to overcome drug resistance in the clinic. Biochim Biophys Acta 2009;1796:75-90. [PubMed]
- Sabbah M, Emami S, Redeuilh G, et al. Molecular signature and therapeutic perspective of the epithelial-to-mesenchymal transitions in epithelial cancers. Drug Resist Updat 2008;11:123-51. [Crossref] [PubMed]


