
Imaging study for colorectal liver metastasis: beyond the diagnosis and to the prognosis
The liver is the most common metastatic site for colorectal cancers, and approximately 15–20% of the patients show liver metastasis on initial. Accurate detection and localization of colorectal liver metastases (CRLM) are crucial to the management of patients with colorectal cancer because CRLM is an important prognostic factor, and its surgical resection can improve the survival. Imaging studies, such as computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET) play an essential role in the detection and localization of CRLM. With technical advances in these imaging studies, such as multi-detector row CT, diffusion-weighted image (DWI), liver MRI using hepatocyte-specific contrast agents, and fluorine-18 fluorodeoxyglucose (FDG) PET fused with CT, their diagnostic performance has improved.
Of these imaging studies, liver MRI with administration of hepatocyte-specific contrast agent, such as gadoxetate disodium (Eovist/Primovist; Bayer Health Care, Berlin, Germany), has the highest sensitivity for the detection of CRLM, which was 93.1% in a recent meta-analysis (1). Notably, liver MRI can be useful in the diagnosis of CRLM with lesions smaller than 1 cm, which are not detected on CT (Figure 1). This high sensitivity can be explained by high lesion conspicuity of CRLM in the hepatobiliary phase (HBP) on gadoxetate disodium-enhanced MRI, indicating hepatocyte-deficient tumors, such as CRLM, which show hypointensity in a background of the hyperintense liver. In addition, DWI can provide additional values for detecting CRLM. In a combination of DWI and HBP image, both techniques are complementary to minimizing the disadvantages of individual techniques, i.e., DWI helped identify metastases located close to the intrahepatic vasculature, while the anatomical details provided by gadoxetate disodium-enhanced MRI were advantageous for regions prone to DWI artifacts or in the liver periphery (2).
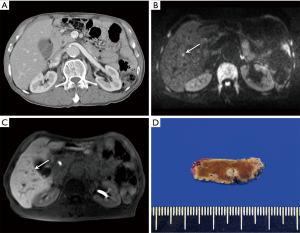
These improvements in the diagnosis of CRLM using imaging studies can affect the treatment plan in patients with CRLM. After the initial CT, a change was made in the treatment plan of 16.8% of the patients owing to findings on gadoxetate disodium-enhanced MRI. These changes included both preventing unnecessary surgery and extending the surgical plan (3). A randomized multi-center study reported that the surgical plan was less modified with gadoxetate disodium-enhanced MRI than with contrast-enhanced CT (28% vs. 47%) (4). In addition, gadoxetate disodium-enhanced MRI might be an independent prognostic factor in patients with CRLM. Kim et al. reported that patients who underwent both CT and MRI had a higher 5-year survival rate than those who underwent CT alone (70.8% vs. 48.1%) (5). These results suggest that gadoxetate disodium-enhanced MRI can be clinically useful in detecting CRLM more accurately, leading to modifications in the treatment plan and increased survival in patients with CRLM.
Another important role of imaging studies in patients with CRLM is assessment of the treatment response. Recently, adding biological agents with an antiangiogenic action (e.g., bevacizumab) to conventional chemotherapy (e.g., FOLFOX or FOLFIRI) increased the overall survival in patients with CRLM. Given these clinical backgrounds, the use of quantitative imaging for assessing the tumor response based on tumor vascularity or cellularity is evolving. Moreover, these new imaging methods can act as imaging biomarkers to predict the response before the initiation of chemotherapy to adapt and optimize the neoadjuvant chemotherapy based on the anticipated treatment response. Several promising tools, including CT attenuation and texture analysis, dynamic contrast-enhanced (DCE) MRI, and intravoxel incoherent motion (IVIM) MRI have been suggested as imaging biomarkers. Notably, DCE MRI can help predict the treatment response through the measurement of capillary perfusion, which determines the delivery of drugs to the tumor cells, and IVIM MRI can be used to assess both diffusion and perfusion changes by analyzing the signal decay curve obtained from multiple b-values. However, as the reported data of these imaging biomarkers for the prediction of response to chemotherapy in CRLM are sparse and heterogeneous, further studies are required to validate and determine the reliable imaging biomarkers to predict the treatment response.
Imaging studies for CRLM are advancing. First, fully integrated FDG PET/MRI has been introduced, which shows an overall good diagnostic performance that is comparable to gadoxetate disodium-enhanced MRI. As FDG PET/MRI can provide information on the overall tumor burden and extrahepatic metastasis, as well as yield a good diagnostic performance in the detection of CRLM (6), FDG PET/MRI can serve as “all-in-one” imaging package in patients with CRLM. In addition, FDG PET/MRI is a promising imaging modality for predicting the prognosis after the neoadjuvant chemotherapy followed by hepatic resection for CRLM (6). Second, the abbreviated MRI protocol, including T2-weighted imaging, DWI, and HBP image, might be an alternative to the standard MRI protocol in CRLM surveillance. As abbreviated MRI can reduce the scan time and cost without significant differences in lesion detection or characterization, it might be accepted and widely used in the clinical practice. The image sequence to be included in abbreviated MRI is not yet determined, and further studies are warranted. Third, radiomics, which is an emerging technique to quantify imaging data with the aid of advanced image processing techniques, has been introduced in the field of colorectal cancers. Several recent studies demonstrated the possibility of predicting the risk of CRLM development and the outcome of patients with CRLM treated with chemotherapy (7).
In conclusion, imaging studies in patients with CRLM are essential in terms of staging, treatment planning, assessing the treatment response, and predicting outcomes. In the past, imaging studies have been developed to improve the diagnostic performance for CRLM. Currently, imaging studies are progressively evolving to serve as prognostic biomarkers and provide an accurate diagnostic examination.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Choi SH, Kim SY, Park SH, et al. Diagnostic performance of CT, gadoxetate disodium-enhanced MRI, and PET/CT for the diagnosis of colorectal liver metastasis: Systematic review and meta-analysis. J Magn Reson Imaging 2018;47:1237-50. [Crossref] [PubMed]
- Koh DM, Collins DJ, Wallace T, et al. Combining diffusion-weighted MRI with Gd-EOB-DTPA-enhanced MRI improves the detection of colorectal liver metastases. Br J Radiol 2012;85:980-9. [Crossref] [PubMed]
- Vreugdenburg TD, Ma N, Duncan JK, et al. Comparative diagnostic accuracy of hepatocyte-specific gadoxetic acid (Gd-EOB-DTPA) enhanced MR imaging and contrast enhanced CT for the detection of liver metastases: a systematic review and meta-analysis. Int J Colorectal Dis 2016;31:1739-49. [Crossref] [PubMed]
- Zech CJ, Korpraphong P, Huppertz A, et al. Randomized multicentre trial of gadoxetic acid-enhanced MRI versus conventional MRI or CT in the staging of colorectal cancer liver metastases. Br J Surg 2014;101:613-21. [Crossref] [PubMed]
- Kim C, Kim SY, Kim MJ, et al. Clinical impact of preoperative liver MRI in the evaluation of synchronous liver metastasis of colon cancer. Eur Radiol 2018;28:4234-42. [Crossref] [PubMed]
- Lee DH, Lee JM, Hur BY, et al. Colorectal cancer liver metastases: diagnostic performance and prognostic value of PET/MR imaging. Radiology 2016;280:782-92. [Crossref] [PubMed]
- Dohan A, Gallix B, Guiu B, et al. Early evaluation using a radiomic signature of unresectable hepatic metastases to predict outcome in patients with colorectal cancer treated with FOLFIRI and bevacizumab. Gut 2019. [Epub ahead of print]. [Crossref] [PubMed]


