
Independent regulation of tumorigenesis and fibrosis in non-alcoholic fatty liver disease
Primary liver cancer, of which hepatocellular carcinoma (HCC) represents the vast majority of cases, is the second highest cause of cancer-related death world-wide (1). HCC typically occurs in the context of chronic liver injury due to chronic diseases, including: viral hepatitis; alcoholic liver disease and; obesity-related non-alcoholic fatty liver disease (NAFLD) and its progressive, inflammatory form; non-alcoholic steatohepatitis (NASH). Due to the liver’s unique regenerative ability, chronic injury is coupled with chronic repair, which, over time, leads to the accumulation of repair-related deposition of extracellular matrix (ECM) proteins, resulting in liver fibrosis. Fibrosis is mediated primarily by the proliferation of the normally quiescent hepatic stellate cells (HSCs) and their secretion of ECM proteins. Because HCC occurrence generally coincides with fibrosis and cirrhosis, it has commonly been accepted that these phenomena play a role in HCC progression. However, while viral hepatitis was classically the major cause of HCC, and can occur almost exclusively in parallel with fibrosis, NAFLD is emerging as a major determinant of HCC due to rising global obesity, and can occur in the absence of fibrosis and cirrhosis (2).
A recent article by Grohmann et al. (3) has questioned the current view that fibrosis and indeed NASH as well are necessary precursors for the development and subsequent progression of HCC during obesity. They showed that obesity leads to the oxidative deactivation of intracellular T cell protein tyrosine phosphatase (TCPTP). TCPTP is a negative regulator of STAT (Signal Transducer and Activator of Transcription) signalling, and thus, its deactivation results in increased STAT-1 and STAT-3 signalling. Furthermore, the genetic deletion of TCPTP, and the resulting STAT-1/3 activity was found to promote NASH-related HCC with fibrosis. Importantly, using heterozygous gene knockout models of STAT-1 and STAT-3 to independently reduce STAT-1 or STAT-3 expression, they found that STAT-1 was required for NASH and fibrosis development but not HCC, and, in contrast, that STAT-3 promoted HCC but not NASH or fibrosis (Figure 1). Taken together, these data suggest that, during obesity, NASH and fibrosis are not accompanying precursors for HCC, and that, instead, HCC and NASH/fibrosis can be driven independently from common initiating factors.
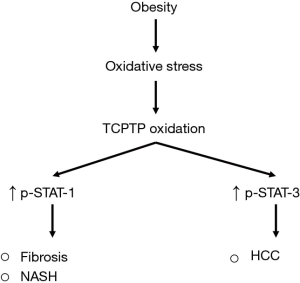
These findings raise important questions about HCC progression in patients with fatty liver disease. These are:
- Are fibrosis and NASH theoretically necessary precursors to HCC?
- Can the functions of STAT-1 and STAT-3 explain these independent events?
- How does HCC in the absence of fibrosis differ and can this be used clinically?
Firstly, fibrosis can promote HCC through the induction of immunosuppressive lymphocytes which express inhibitory programmed death-ligand 1 (PD-L1) receptors, leading to the suppression of CD8+ T-cell-mediated immunosurveillance (4). However, data also supports the concept that obesity-driven HCC can occur without fibrosis (2). Thus, these HCCs clearly arise through different mechanisms. The inflammatory environment of NASH, which separates it from the less progressive NAFLD, plays an even more crucial role in promoting HCC. Here, pathogenesis is mediated through mechanisms that include the induction of endoplasmic reticulum (ER) stress and tumour necrosis factor (TNF)-mediated liver damage and promotion of HCC progenitor cell proliferation, as well as genomic instability (4). Thus, an explanation of how HCC can occur without NASH is lacking in the field, and the study by Grohmann et al. goes a small way to suggest that STAT-3 may be one of these causative factors.
Secondly, the reviewed work differs from expectations in the field (3). STAT proteins are transcription factors which act downstream of cytokines and growth factors. STAT-1 and STAT-3 are known to have almost entirely opposite functions, even though they share many common regulators and expression targets. STAT-1 is a tumour-suppressor which typically promotes apoptosis and immune cell recruitment, while STAT-3 is an oncogene which promotes proliferation and immune suppression (5). Therefore, these known functions support a role for STAT-1 promoting NASH which involves immune cell infiltration into the liver, and a role for STAT-3 in promoting HCC. However, other observations by Grohmann et al. contradict these functions. For example, fibrosis involves proliferation of HSCs, which goes against the known anti-proliferative functions of STAT-1. Indeed, previous studies found that STAT-1 inhibits liver fibrosis (6). In addition, it has been shown that the anti-fibrotic effects of sorafenib are in part due to the down-regulation of STAT-3, and that STAT-3 overexpression can enhance HSC proliferation (7). Hence, further analyses are required to illicit the independent regulation of NASH and fibrosis in HCC.
Given such discrepancies, a feature of the study which requires further consideration is the reciprocal regulation of STAT-1 and STAT-3. STAT-1 and STAT-3 are known to regulate each other (5). However, it remains unclear from Grohmann et al.’s study whether the correction of either STAT-1 or STAT-3 signalling affected the other. Indeed, they found that correcting STAT-3 signalling lowered interferon-γ (IFNγ) levels, a major inducer of STAT-1. In contrast, STAT-1 correction did not significantly alter interleukin-6 (IL-6) levels, the major inducer of STAT-3. Thus, this potentially confounding factor requires further assessment in future studies.
Finally, understanding the differences between HCC that occurs in the presence or absence of fibrosis could have important impacts treatment approaches. The view that HCC occurs subsequently to fibrosis means that surveillance systems are limited to patients presenting with these features alone. Worryingly, more than 40% of men with HCC may develop tumours in the absence of fibrosis (2). Thus, surveillance systems need to be re-evaluated to be brought in-line with current knowledge. Furthermore, while these tumours tend to be larger, they also have significantly lower rates of recurrence after surgical resection or radiofrequency ablation (2). Thus, surgical therapy could be considered for the treatment of these HCCs. Moreover, further studies are required to determine their genetic and protein signaling promoters.
While future research is required to determine whether STAT-1 and STAT-3 are the true molecular switch between HCC and fibrosis and NASH, it is clear that these events can occur independently. This has been suspected in human patients (2), and has now been demonstrated experimentally. This finding has major implications to both the surveillance mechanisms used to assess risk of HCC development, and for the development of drugs to treat liver disease.
Acknowledgments
Funding: This work was supported by Cancer Council Queensland Project Grant (1123436, L Hebbard).
Footnote
Conflict of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2095-128. [Crossref] [PubMed]
- Kodama K, Kawaguchi T, Hyogo H, et al. Clinical features of hepatocellular carcinoma in nonalcoholic fatty liver disease patients without advanced fibrosis. J Gastroenterol Hepatol 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Grohmann M, Wiede F, Dodd GT, et al. Obesity Drives STAT-1-Dependent NASH and STAT-3-Dependent HCC. Cell 2018;175:1289-306.e20. [Crossref] [PubMed]
- Karin M. New insights into the pathogenesis and treatment of non-viral hepatocellular carcinoma: a balancing act between immunosuppression and immunosurveillance. Precis Clin Med 2018;1:21-8. [Crossref] [PubMed]
- Regis G, Pensa S, Boselli D, et al. Ups and downs: the STAT1:STAT3 seesaw of Interferon and gp130 receptor signalling. Semin Cell Dev Biol 2008;19:351-9. [Crossref] [PubMed]
- Jeong WI, Park O, Radaeva S, et al. STAT1 inhibits liver fibrosis in mice by inhibiting stellate cell proliferation and stimulating NK cell cytotoxicity. Hepatology 2006;44:1441-51. [Crossref] [PubMed]
- Su TH, Shiau CW, Jao P, et al. Sorafenib and its derivative SC-1 exhibit antifibrotic effects through signal transducer and activator of transcription 3 inhibition. Proc Natl Acad Sci U S A 2015;112:7243-8. [Crossref] [PubMed]


