Should preoperative biliary drainage be routinely performed for obstructive jaundice with resectable tumor?
Obstructive jaundice is a common clinical manifestation of malignancy in extrahepatic bile duct, ampulla or pancreas. When obstructive jaundice happens, symptoms and signs such as yellow skin and sclera, skin itching, dark urine, and light color stool will present accompanied by progressive liver damage and even liver failure. For jaundice caused by unresectable tumors, options usually include bypass surgery, endoscopic biliary stent placement, percutaneous stent placement, and chemotherapy (1). However, for jaundice caused by resectable tumors, radical surgery is the only effective treatment. In general, obstruction can be divided into the upper and the lower obstruction based on its location. For the upper obstruction, complete resection of cholangiocarcinoma in combination with partial hepatectomy is often required. In case of the lower obstruction, pancreaticoduodenectomy is usually performed. Given the facts that patients with obstructive jaundice often have poor nutritional status, severe liver dysfunction, and perioperative complications, a practical consideration is to improve patient tolerance of surgery by reducing preoperative bilirubin levels. However, whether there should be a routine preoperative biliary drainage remains controversial. It is still debatable on whether the procedure truly benefits the patients. In this paper, we reviewed recently published literatures with an aim to further analyze and discuss the dispute.
Concept of the preoperative biliary drainage
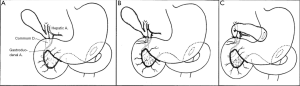
In 1935, Whipple et al. suggested that poor nutritional status in patients with obstructive jaundice and severe liver damage may greatly affect patient’s tolerance to a one-step removal of ampullary tumors. A staged surgery, which consists of gallbladder gastric anastomosis (Figure 1) followed by tumor resection 3-4 weeks afterwards, was proposed (2). The concept of preoperative biliary drainage, therefore, was first established.
Methods for preoperative biliary drainage
In the early days, biliary drainage was achieved mainly through surgery (2,3). In the 1950s Carter et al. brought percutaneous transhepatic cholangiography (PTC) technology into the clinical application (4). In late 1960s, McCune proposed endoscopic retrograde cholangiopancreatography (ERCP) technique (5), which significantly reduced the surgical trauma of preoperative biliary drainage. The procedure can be divided into internal drainage and external drainage, referring to the bile being drained to the inside and outside of the intestine respectively. Major complications related to the procedure consist of pancreatitis, cholangitis, perforation, bleeding, and stent restructure (6). Comparing with ERCP, PTC generates fewer complications, and is superior in the speed of bilirubin reduction (7), and has a relatively lower cost (8). ERCP may lead to the emergence of duodenal flora migration that could result in cholangitis, especially in the cases of proximal obstruction; therefore it is not the first choice (9). Although PTC seems to be advantageous over ERCP, there has been no randomized controlled clinical study for a solid conclusion. The choice of methods relies mainly on the experience of individual professional.
Efficacy of preoperative biliary drainage
The benefits of biliary drainage were mainly observed in animal studies. Studies have shown that obstructive jaundice in animal models leads to lack of bile salts in the digestive tract, disorder of intestinal flora, and the increase of endotoxin concentration in portal system. It also causes damage to intestinal barrier which leads to the increase of mucosal permeability (10-12). The function of Kupffer cells can also be impaired, so is the cellular immune function due to the increased levels of tumor necrosis factor (TNF), interleukin-6 (IL6) and other inflammatory cytokines (13-16); increasing the probability of bacterial infection and tumor metastasis (17,18). However, all these effects are mainly associated with elevated endotoxin levels (19), and have little to do with hyperbilirubinemia (15). Intestinal barrier dysfunction may be caused by significantly decreased intestinal epithelial cell proliferation and the disruption of tight junction protein expression during obstructive jaundice (20,21). Several natural and synthetic reagents, including turmeric, glutamate (22), Lactobacillus plantarum (23), and Astragalus (24), were shown to protect small intestine and its barrier function, thereby reducing inflammation.
Studies have shown that after undergoing biliary drainage procedure, liver function was significantly improved, endotoxemia reduced, cytokine release decreased (16,25), and immune function restored (26). The internal drainage is significantly better than external drainage in reducing endotoxemia (16). Result could be more satisfied by applying Lactobacillus plantarum with the internal drainage (27).
Clinically, it was found that hepatocellular apoptosis and bile lake were reduced after biliary drainage procedure (28), whereby liver function was protected. The surgical delay of 4-6 weeks due to biliary drainage procedure did not significantly affect survival (29).
In addition to liver function, other organ function can be impacted by obstructive jaundice as well. The elevated bile acid level inhibits hepatic glucocorticoid metabolism, suppresses the hypothalamic-pituitary-adrenal axis activity (30). Clinical studies found that levels of atrial natriuretic peptide (ANP) increased in obstructive jaundice patients (31), and decreased following the internal drainage, indicating the improved heart function (32). Kidney failure is another common complication of obstructive jaundice (33,34) with acute tubular necrosis and venous dilatation as pathological manifestations (35). Rehydration therapy alone often cannot relieve such renal failure. Either internal drainage (36) or external drainage (34) may lead to significant improvement of renal function.
Although it is evident that biliary drainage brings favorable outcome pathophysiologally, it is not a clear cut when the clinical perioperative morbidity and mortality are considered. Surgical complications often include pancreatic anastomotic fistula, postoperative bleeding, delayed gastric emptying, biliary fistula, gastrojejunostomy anastomotic fistula, intra-abdominal abscess, wound infection, portal vein thrombosis, pneumonia, cholangitis, and myocardial infarction (6).
During 1980s, several randomized controlled clinical studies found that the external drainage would not improve prognosis, but rather increase the incidence of complications (37-39), thus raising the hospitalization and costs (39). However, questions were also raised about credibility of these studies. Pisters et al. argued that the Evidence-Based Medicine concept was only well established after the mid-1990s, and considered these studies not performed methodological strictly. They believed that these studies employed outdated technology that resulted in low resection rate (16%) and high perioperative mortality (12%). Therefore, these findings needed to be reviewed carefully (40). Meanwhile, their own retrospective study indicated that preoperative biliary drainage (stenting) increased the risk of postoperative wound infection, but not the risk of major postoperative complications or mortality (41). Povoski et al. reported that preoperative biliary drainage is the only factor associated with postoperative infection, intra-abdominal abscess, and postoperative mortality. The preoperative biliary drainage should therefore be avoided for resectable pancreatic head tumor or other adjacent tumors (42). van der Gaag reported in a randomized controlled study of pancreatic head cancer that the preoperative biliary drainage group (either ERCP or PTC) incurred 47% postsurgical complications in contrast to the 37% in the direct surgery group. Thus, preoperative biliary drainage is not recommended for obstructive jaundice caused by pancreatic head mass (6). Meta-analysis by some scholars has found that although preoperative biliary drainage induces higher incidences of complications it does not affect postoperative mortality. There is no definite evidence to support or against preoperative biliary drainage (43-45).
The benefits in pathophysiology and the adversities in perioperative complications for preoperative biliary drainage are clearly hard to reconcile. One of the possible explanations can be that the animal model of obstructive jaundice was obtained through bile duct ligation which caused acute obstructive jaundice. The manifestations of the animal model and their pathophysiological process can be very different from the chronic progressive jaundice caused by malignant obstructive. Although several clinical studies did point out the benefits of preoperative biliary drainage in patients concerning pathophysiology (32,34-36), overwhelming evidences from randomized, controlled clinical studies pointed to the unfavorable outcomes in perioperative complications caused by the procedure. Taking all current domestic and international clinical studies into consideration, we believe that malignant obstructive jaundice does not require routine preoperative biliary drainage (Figure 2).
Conclusions
Currently, there is still no definite conclusion on whether it is needed for preoperative jaundice alleviation through biliary drainage. Although the benefit in pathophysiology is evident, the clinical studies have revealed many adversities for this procedure. In light of currently available information, we believe that the adversities in perioperative complications resulted from preoperative biliary drainage clearly outweighs its benefits in alleviating pathophysiological symptoms. Thus, we believe that obstructive jaundice does not require routine preoperative biliary drainage. However, large-scale clinical randomized, controlled, prospective studies are still needed for a clear and un-ambivalent answer.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Mihalache F, Tantău M, Iancu C, et al. Therapeutic approach to the malignant tumors of the biliary tract. Rom J Intern Med 2010;48:131-40. [PubMed]
- Whipple AO, Parsons WB, Mullins CR. Treatment of carcinoma of the ampulla of vater. Ann Surg 1935;102:763-79. [PubMed]
- Maki T, Sato T, Kakizaki G, et al. Pancreatoduodenectomy for periampullary carcinomas. Appraisal of two-stage procedure. Arch Surg 1966;92:825-33. [PubMed]
- Carter RF, Saypol GM. Transabdominal cholangiography. J Am Med Assoc 1952;148:253-5. [PubMed]
- McCune WS, Shorb PE, Moscovitz H. Endoscopic cannulation of the ampulla of vater: a preliminary report. Ann Surg 1968;167:752-6. [PubMed]
- van der Gaag NA, Rauws EA, van Eijck CH, et al. Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med 2010;362:129-37. [PubMed]
- Park SY, Park CH, Cho SB, et al. What is appropriate procedure for preoperative biliary drainage in patients with obstructive jaundice awaiting pancreaticoduodenectomy? Surg Laparosc Endosc Percutan Tech 2011;21:344-8. [PubMed]
- Hong SK, Jang JY, Kang MJ, et al. Comparison of clinical outcome and cost-effectiveness after various preoperative biliary drainage methods in periampullary cancer with obstructive jaundice. J Korean Med Sci 2012;27:356-62. [PubMed]
- Maguchi H, Takahashi K, Katanuma A, et al. Preoperative biliary drainage for hilar cholangiocarcinoma. J Hepatobiliary Pancreat Surg 2007;14:441-6. [PubMed]
- Parks RW, Clements WD, Smye MG, et al. Intestinal barrier dysfunction in clinical and experimental obstructive jaundice and its reversal by internal biliary drainage. Br J Surg 1996;83:1345-9. [PubMed]
- Clements WD, McCaigue M, Erwin P, et al. Biliary decompression promotes Kupffer cell recovery in obstructive jaundice. Gut 1996;38:925-31. [PubMed]
- Tanaka N, Ryden S, Bergqvist L, et al. Reticulo-endothelial function in rats with obstructive jaundice. Br J Surg 1985;72:946-9. [PubMed]
- Kennedy JA, Clements WD, Kirk SJ, et al. Characterization of the Kupffer cell response to exogenous endotoxin in a rodent model of obstructive jaundice. Br J Surg 1999;86:628-33. [PubMed]
- Sewnath ME, van der Poll T, van Noorden CJ, et al. Cholestatic interleukin-6-deficient mice succumb to endotoxin-induced liver injury and pulmonary inflammation. Am J Respir Crit Care Med 2004;169:413-20. [PubMed]
- Greve JW, Gouma DJ, Soeters PB, et al. Suppression of cellular immunity in obstructive jaundice is caused by endotoxins: a study with germ-free rats. Gastroenterology 1990;98:478-85. [PubMed]
- Gouma DJ, Coelho JC, Fisher JD, et al. Endotoxemia after relief of biliary obstruction by internal and external drainage in rats. Am J Surg 1986;151:476-9. [PubMed]
- Kawarabayashi N, Seki S, Hatsuse K, et al. Immunosuppression in the livers of mice with obstructive jaundice participates in their susceptibility to bacterial infection and tumor metastasis. Shock 2010;33:500-6. [PubMed]
- Lane DR, Joshi P, Grogan JB, et al. Suppression of natural killer cell activity in biliary obstruction. Am Surg 1996;62:259-62. [PubMed]
- Jones C, Badger SA, Black JM, et al. The use of antiendotoxin peptides in obstructive jaundice endotoxemia. Eur J Gastroenterol Hepatol 2012;24:248-54. [PubMed]
- Assimakopoulos SF, Tsamandas AC, Louvros E, et al. Intestinal epithelial cell proliferation, apoptosis and expression of tight junction proteins in patients with obstructive jaundice. Eur J Clin Invest 2011;41:117-25. [PubMed]
- Wang N, Yu H, Ma J, et al. Evidence for tight junction protein disruption in intestinal mucosa of malignant obstructive jaundice patients. Scand J Gastroenterol 2010;45:191-9. [PubMed]
- Karatepe O, Acet E, Battal M, et al. Effects of glutamine and curcumin on bacterial translocation in jaundiced rats. World J Gastroenterol 2010;16:4313-20. [PubMed]
- Zhang M, Wang XQ, Zhou YK, et al. Effects of oral Lactobacillus plantarum on hepatocyte tight junction structure and function in rats with obstructive jaundice. Mol Biol Rep 2010;37:2989-99. [PubMed]
- Xiping Z, Ke W, Yaping Y, et al. Protective effect and mechanisms of radix astragali injection on the intestinal mucosa of rats with obstructive jaundice. Mediators Inflamm 2010;2010:757191.
- Roughneen PT, Gouma DJ, Kulkarni AD, et al. Impaired specific cell-mediated immunity in experimental biliary obstruction and its reversibility by internal biliary drainage. J Surg Res 1986;41:113-25. [PubMed]
- Megison SM, Dunn CW, Horton JW, et al. Effects of relief of biliary obstruction on mononuclear phagocyte system function and cell mediated immunity. Br J Surg 1991;78:568-71. [PubMed]
- Zhou YK, Qin HL, Zhang M, et al. Effects of Lactobacillus plantarum on gut barrier function in experimental obstructive jaundice. World J Gastroenterol 2012;18:3977-91. [PubMed]
- Lalisang TJ, Sjamsuhidajat R, Siregar NC, et al. Profile of hepatocyte apoptosis and bile lakes before and after bile duct decompression in severe obstructive jaundice patients. Hepatobiliary Pancreat Dis Int 2010;9:520-3. [PubMed]
- Eshuis WJ, van der Gaag NA, Rauws EA, et al. Therapeutic delay and survival after surgery for cancer of the pancreatic head with or without preoperative biliary drainage. Ann Surg 2010;252:840-9. [PubMed]
- McNeilly AD, Macfarlane DP, O’Flaherty E, et al. Bile acids modulate glucocorticoid metabolism and the hypothalamic-pituitary-adrenal axis in obstructive jaundice. J Hepatol 2010;52:705-11. [PubMed]
- Martínez-Ródenas F, Pereira JA, Jiménez W, et al. Circulating bile is the main factor responsible for atrial natriuretic peptide release in experimental obstructive jaundice. Br J Surg 1998;85:480-4. [PubMed]
- Padillo J, Puente J, Gómez M, et al. Improved cardiac function in patients with obstructive jaundice after internal biliary drainage: hemodynamic and hormonal assessment. Ann Surg 2001;234:652-6. [PubMed]
- Wait RB, Kahng KU. Renal failure complicating obstructive jaundice. Am J Surg 1989;157:256-63. [PubMed]
- Parildar Z, Cinar C, Barutçuoğlu B, et al. Effects of percutaneous transhepatic biliary drainage on renal function in patients with obstructive jaundice. Diagn Interv Radiol 2011;17:74-9. [PubMed]
- Uslu A, Taşli FA, Nart A, et al. Human kidney histopathology in acute obstructive jaundice: a prospective study. Eur J Gastroenterol Hepatol 2010;22:1458-65. [PubMed]
- Padillo FJ, Briceño J, Cruz A, et al. Randomized clinical trial of the effect of intravenous fluid administration on hormonal and renal dysfunction in patients with obstructive jaundice undergoing endoscopic drainage. Br J Surg 2005;92:39-43. [PubMed]
- Hatfield AR, Tobias R, Terblanche J, et al. Preoperative external biliary drainage in obstructive jaundice. A prospective controlled clinical trial. Lancet 1982;2:896-9. [PubMed]
- McPherson GA, Benjamin IS, Hodgson HJ, et al. Pre-operative percutaneous transhepatic biliary drainage: the results of a controlled trial. Br J Surg 1984;71:371-5. [PubMed]
- Pitt HA, Gomes AS, Lois JF, et al. Does preoperative percutaneous biliary drainage reduce operative risk or increase hospital cost? Ann Surg 1985;201:545-53. [PubMed]
- Pisters PW, Lee JE, Vauthey JN, et al. Comment and perspective on Sewnath and colleagues’ recent meta-analysis of the efficacy of preoperative biliary drainage for tumors causing obstructive jaundice. Ann Surg 2003;237:594-5; author reply 595-6. [PubMed]
- Pisters PW, Hudec WA, Hess KR, et al. Effect of preoperative biliary decompression on pancreaticoduodenectomy-associated morbidity in 300 consecutive patients. Ann Surg 2001;234:47-55. [PubMed]
- Povoski SP, Karpeh MS Jr, Conlon KC, et al. Association of preoperative biliary drainage with postoperative outcome following pancreaticoduodenectomy. Ann Surg 1999;230:131-42. [PubMed]
- Qiu YD, Bai JL, Xu FG, et al. Effect of preoperative biliary drainage on malignant obstructive jaundice: a meta-analysis. World J Gastroenterol 2011;17:391-6. [PubMed]
- Liu F, Li Y, Wei Y, et al. Preoperative biliary drainage before resection for hilar cholangiocarcinoma: whether or not? A systematic review. Dig Dis Sci 2011;56:663-72. [PubMed]
- Fang Y, Gurusamy KS, Wang Q, et al. Pre-operative biliary drainage for obstructive jaundice. Cochrane Database Syst Rev 2012;9:CD005444. [PubMed]


