Carotenoids and alcoholic liver disease
Excess consumption of alcohol is commonplace in the United States and worldwide and is known to cause serious organ damage. Specifically, alcohol consumption is one of the most prominent factors contributing to liver disease—a major cause of morbidity and mortality worldwide (1). Alcoholic liver disease (ALD) includes a broad spectrum of disease stages beginning with steatosis (fatty liver) and followed by alcoholic steatohepatitis. These lay the groundwork for progression to the more damaging and irreversible stages of fibrosis and cirrhosis, which increases the risk of hepatocellular carcinoma development (2).
β-Carotene is a provitamin A carotenoid found in many fruits and vegetables that is known to possess potent antioxidant functions (3). In a recent issue of Hepatobiliary Surgery and Nutrition, Peng et al. explored the effects of β-carotene supplementation on antioxidant capacity and hepatic apoptosis in a chronic ethanol-fed rat model (4). The study supplemented with low dose (0.52 mg/kg BW/day) and high dose (2.6 mg/kg BW/day) β-carotene in rats fed either Lieber-DeCarli control or 35% ethanol diet for a treatment period of 12 weeks. Analysis revealed that consumption of the ethanol diet resulted in hepatic injury including elevated plasma AST and ALT activities, fatty liver, plasma and hepatic TNFα concentrations, lipid peroxidation, cytochrome P450 2E1 (CYP2E1) protein expression, and apoptosis. Interestingly, β-carotene supplementation provided a hepatoprotective effect against the ethanol-induced hepatic injury seen in the rat model. Both supplementation doses prevented ethanol-induced liver damage and were associated with a significant reduction in liver injury markers AST and ALT. An important finding in this paper was that the two doses of β-carotene supplementation appeared to work via different mechanistic targets. Specifically, the hepatoprotective actions of low dose (0.52 mg/kg BW/day) β-carotene supplementation were associated with the inhibition of mitochondrial-mediated apoptosis via increased Bcl-xL and decreased caspase-3 and -9 hepatic protein expressions. Low dose β-carotene supplementation was also associated with lowered oxidative stress as shown by decreased lipid peroxidation and CYP2E1 protein expression levels. On the other hand, the hepatoprotective actions of high dose (2.6 mg/kg BW/day) β-carotene supplementation were associated with decreased plasma and hepatic TNFα concentrations and lipid peroxidation. Additionally, high dose β-carotene supplementation resulted in decreased cytochrome c and increased Bcl-2 protein expression (4). In summary, these data provide evidence that supplementation with β-carotene exerts protective effects against alcohol-induced hepatic injury.
Low dose β-carotene supplementation studies have previously demonstrated a beneficial effect against alcohol-induced liver injury via antioxidant activity (5,6). In contrast, high dose β-carotene was shown to have no beneficial antioxidant effect, and was even proposed to act as a prooxidant in the presence of alcohol (7). Indeed, high dose β-carotene supplementation intensified liver damage in an alcohol model (8). Supplementation with β-carotene has also been shown to potentiate alcohol-induced CYP2E1 levels in rat livers, which was confirmed by the corresponding increase in the hydroxylation of p-nitrophenol, a specific substrate for the CYP2E1 enzyme (9). As mentioned, Peng et al. demonstrated a decrease in lipid peroxidation with both low (0.52 mg/kg BW/day) and high (2.6 mg/kg BW/day) doses of β-carotene supplementation and a significant decrease in CYP2E1 protein expression with low dose β-carotene supplementation only (4). It is important to note that although this study utilized both low and high supplementation doses, both are considerably lower than the doses found to have detrimental effects in the presence of alcohol. These findings emphasize the importance of low dose β-carotene supplementation with alcohol consumption.
An interesting addition to this study would be to address the possibility that the hepatoprotective effects in the study are due to the conversion of the β-carotene supplement into vitamin A and retinoic acid, the bioactive form of vitamin A. Retinoic acid functions as a nuclear transcription factor ligand and controls the expression of hundreds of genes (10). Vitamin A can be consumed directly from the diet, usually in the form of retinol or retinyl esters from a variety of meat and dairy products. In addition, it can also be produced via enzymatic cleavage from dietary provitamin A carotenoids (α-carotene, β-carotene, and β-cryptoxanthin). Specifically, β-carotene 15,15'-monooxygenase (Figure 1) has a high affinity for the cleavage of β-carotene to produce retinal (11). Our lab has demonstrated that alcohol consumption results in the up-regulation of β-carotene 15,15'-monooxygenase (12), which supports the previous observation that heavy consumption of alcohol substantially reduces plasma levels of β-carotene in humans (13,14). Further, a previous study providing β-carotene as the major source of vitamin A demonstrated that ablation of β-carotene 15,15'-monooxygenase resulted in a dramatic decrease in vitamin A as well as the development of fatty liver (15). Therefore, it is possible that altered vitamin A levels could be associated with the alcohol-induced liver injury observed by Peng et al. as well (4).
Chronic and excessive alcohol intake is known to interfere with vitamin A homeostasis and metabolism in several different ways (Figure 1) (10,16,17). Ethanol is metabolized primarily in the liver to acetaldehyde via oxidative metabolism, and then converted to acetic acid. The metabolism of vitamin A undergoes a very similar oxidative process including the conversion of retinol to retinal, followed by conversion to the bioactive form, retinoic acid (10). Ethanol and retinol share similar chemical properties, and ethanol has been shown to act as a competitive inhibitor of both ethanol and acetaldehyde dehydrogenases, thus inhibiting vitamin A oxidation to bioactive retinoic acid (16,17). Additionally, induction of the CYP2E1 enzyme by ethanol has been shown to enhance retinol and retinoic acid catabolism (16). Specifically, utilizing the CYP2E1 inhibitor chlormethiazole, we have demonstrated that chlormethiazole normalizes hepatic retinoic acid, retinol, and retinyl ester levels and prevents the appearance of retinoid degradation metabolites in a dose dependent manner (18). Lastly, ethanol has been shown to alter retinoid homeostasis by increasing the mobilization of vitamin A to extrahepatic tissues (10,16).
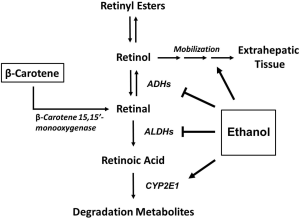
Alcoholics are reported to have significantly reduced levels of plasma retinol as well as hepatic retinyl ester stores (19-21). Specifically, ALD is associated with depleted levels of hepatic vitamin A (17) and the decrease in hepatic retinoid content is correlated with disease severity (22). Further, animal models have demonstrated that alcohol depletes retinoid stores independent of vitamin A intake and absorption (23). Studies in our laboratory have demonstrated that hepatic concentrations of retinoic acid, retinol, and retinyl palmitate are significantly reduced upon high consumption of alcohol (12,24). Interestingly, multiple studies have shown that supplementation with retinoic acid alleviates alcohol-induced liver injury (25-27). Therefore, it is important to point out that if the β-carotene supplementation restored the vitamin A status to within normal range, this may explain the protective effects of β-carotene against the alcohol-related liver injury seen in the study.
In conclusion, examination of the role of β-carotene 15, 15'-monooxygenase activity and vitamin A status in β-carotene supplementation studies will help to provide a more detailed explanation of the involvement of retinoid levels in the protection against alcohol-induced hepatic injury. Further, we suggest a need for caution among individuals consuming high amounts of both alcohol and β-carotene supplements.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- O’Shea RS, Dasarathy S, McCullough AJ, et al. Alcoholic liver disease. Hepatology 2010;51:307-28. [PubMed]
- Gao B, Bataller R.. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology 2011;141:1572-85. [PubMed]
- Miao B, Wang XD. Provitamin A Carotenoids and Cancer Prevention. New York: Springer Science + Business Media, 2013.
- Peng HC, Chen YL, Yang SY, et al. The antiapoptotic effects of different doses of β-carotene in chronic ethanol-fed rats. Hepatobiliary Surg Nutr 2013;2:132-41.
- Werman MJ, Ben-Amotz A, Mokady S. Availability and antiperoxidative effects of beta-carotene from Dunaliella bardawil in alcohol-drinking rats. J Nutr Biochem 1999;10:449-54. [PubMed]
- Lin WT, Huang CC, Lin TJ, et al. Effects of beta-carotene on antioxidant status in rats with chronic alcohol consumption. Cell Biochem Funct 2009;27:344-50. [PubMed]
- Portari GV, Jordão Júnior AA, Meirelles MS, et al. Effect of beta-carotene supplementation on rats submitted to chronic ethanol ingestion. Drug Chem Toxicol 2003;26:191-8. [PubMed]
- Leo MA, Kim C, Lowe N, et al. Interaction of ethanol with beta-carotene: delayed blood clearance and enhanced hepatotoxicity. Hepatology 1992;15:883-91. [PubMed]
- Kessova IG, Leo MA, Lieber CS. Effect of beta-carotene on hepatic cytochrome P-450 in ethanol-fed rats. Alcohol Clin Exp Res 2001;25:1368-72. [PubMed]
- Clugston RD, Blaner WS. The adverse effects of alcohol on vitamin A metabolism. Nutrients 2012;4:356-71. [PubMed]
- von Lintig J.. Provitamin A metabolism and functions in mammalian biology. Am J Clin Nutr 2012;96:1234S-44S. [PubMed]
- Luvizotto RA, Nascimento AF, Veeramachaneni S, et al. Chronic alcohol intake upregulates hepatic expression of carotenoid cleavage enzymes and PPAR in rats. J Nutr 2010;140:1808-14. [PubMed]
- Fukao A, Tsubono Y, Kawamura M, et al. The independent association of smoking and drinking with serum beta-carotene levels among males in Miyagi, Japan. Int J Epidemiol 1996;25:300-6. [PubMed]
- Stryker WS, Kaplan LA, Stein EA, et al. The relation of diet, cigarette smoking, and alcohol consumption to plasma beta-carotene and alpha-tocopherol levels. Am J Epidemiol 1988;127:283-96. [PubMed]
- Hessel S, Eichinger A, Isken A, et al. CMO1 deficiency abolishes vitamin A production from beta-carotene and alters lipid metabolism in mice. J Biol Chem 2007;282:33553-61. [PubMed]
- Bell H, Nilsson A, Norum KR, et al. Retinol and retinyl esters in patients with alcoholic liver disease. J Hepatol 1989;8:26-31. [PubMed]
- Leo MA, Sato M, Lieber CS. Effect of hepatic vitamin A depletion on the liver in humans and rats. Gastroenterology 1983;84:562-72. [PubMed]
- Majumdar SK, Shaw GK, Thomson AD. Vitamin A utilization status in chronic alcoholic patients. Int J Vitam Nutr Res 1983;53:273-9. [PubMed]
- Crabb DW, Pinairs J, Hasanadka R, et al. Alcohol and retinoids. Alcohol Clin Exp Res 2001;25:207S-217S. [PubMed]
- Leo MA, Lieber CS. Hepatic vitamin A depletion in alcoholic liver injury. N Engl J Med 1982;307:597-601. [PubMed]
- Sato M, Lieber CS. Hepatic vitamin A depletion after chronic ethanol consumption in baboons and rats. J Nutr 1981;111:2015-23. [PubMed]
- Wang XD, Liu C, Chung J, et al. Chronic alcohol intake reduces retinoic acid concentration and enhances AP-1 (c-Jun and c-Fos) expression in rat liver. Hepatology 1998;28:744-50. [PubMed]
- Chung J, Chavez PR, Russell RM, et al. Retinoic acid inhibits hepatic Jun N-terminal kinase-dependent signaling pathway in ethanol-fed rats. Oncogene 2002;21:1539-47. [PubMed]
- Chung J, Liu C, Smith DE, et al. Restoration of retinoic acid concentration suppresses ethanol-enhanced c-Jun expression and hepatocyte proliferation in rat liver. Carcinogenesis 2001;22:1213-9. [PubMed]
- Pan Z, Dan Z, Fu Y, et al. Low-dose ATRA supplementation abolishes PRM formation in rat liver and ameliorates ethanol-induced liver injury. J Huazhong Univ Sci Technolog Med Sci 2006;26:508-12. [PubMed]
- Wang XD. Alcohol, vitamin A, and cancer. Alcohol 2005;35:251-8. [PubMed]
- Liu C, Chung J, Seitz HK, et al. Chlormethiazole treatment prevents reduced hepatic vitamin A levels in ethanol-fed rats. Alcohol Clin Exp Res 2002;26:1703-9. [PubMed]


