
Perioperative impact of liver venous deprivation compared with portal venous embolization in patients undergoing right hepatectomy: preliminary results from the pioneer center
Introduction
Post-hepatectomy liver failure (PHLF) represents the most challenging complication after liver resection. Incidence varies in the literature from 0.7% to 35% (1) and it is the main cause of death following major hepatectomies. To date, different techniques and strategies have been proposed to face this severe and life-threatening complication (2). Since its first use in 1984 for a hilar cholangiocarcinoma (3), preoperative portal vein embolization (PVE) is now the standard technique to increase the size of the future remnant liver (FRL)before major hepatectomies or staged bilateral resections (4). The immediate redistribution of portal blood flow is responsible of the hyperplasia of the hepatocytes with a degree of hypertrophy (DH) of the non-embolized segments that stands approximatively at 10% and requires usually up to 4–6 weeks (5,6). Portal vein ligation (PVL) has been equally proposed with a comparable safety and similar morbidity and mortality rates (7,8). Nevertheless, these approaches do not always induce fast and sufficient hypertrophy of the FLR, explaining that 20% of patients will ultimately not be resected (4). In order to face this issue, associated liver partition and portal vein ligation for staged hepatectomy (ALPPS) has been developed. It is associated with a higher and faster DH but, unfortunately, with a higher morbidity and mortality rate, mainly due to increased risk of PHLF (9-11). A method recently proposed to increase the FRL is the liver venous deprivation (LVD) technique (12). It consists of simultaneous embolization of portal and one or two hepatic veins in order to increase the damage to the embolized liver leading to increased hypertrophy of the contralateral parenchyma. Some authors had previously described the embolization of the hepatic vein 1 to 3 weeks after portal vein occlusion in case of extended disease of insufficient liver hypertrophy (13-16). This strategy surprisingly generated a further rise of the FRL. Very recently, portal and hepatic vein ligation was tested in pigs resulting in optimal tolerance and higher liver hypertrophy compared with portal vein ligation alone (17). In two series, the same results were achieved with promising prospects (18,19), but surgical related morbidity and mortality after LVD remained to be explored. Furthermore, histological findings after portal and hepatic vein occlusion were different from those after PVE, with more marked hepatocellular damage and sinusoidal dilatation. The resulting vascular congestion and the possibility of veno-venous shunts creation could potentially lead to an increased risk of intra-operative bleeding. For all these reasons, we report our series of hepatic resections after PVE and LVD. The aim of this study is to compare intra- and post-operative morbidity and mortality between the two techniques in patients undergoing right hepatectomy. The secondary endpoint is evaluation of the histological specimens in order to compare the morphological alteration of hepatocytes and sinusoidal endothelial cells between the two groups.
Methods
Study design
This is a mono-institutional observational retrospective study. An informed consent was acquired before both the embolization and the surgical operation. This study was approved by the Institutional Review Board (#2019-68) and it was aligned to Helsinki declaration. In addition, it has been conducted according to the Strengthening the Reporting of Observation Studies in Epidemiology (STROBE) guidelines of the EQUATOR network (20).
Patient selection and preoperative work-out
Fifty-three consecutive patients undergoing PVD and LVD before major hepatectomy between April 2015 and December 2017 were retrospectively analysed (Figure 1). Data included potential right hepatectomies (n=29), right trisectionectomies (n=9), left hepatectomy (n=1) or other major hepatectomies (n=14, right hepatectomies or trisectionectomies associated with wedge resections of the left liver). In order to reduce confounding variables related to the nature of the hepatic resection only patients undergoing potential standard right hepatectomy have been considered. Both radiological and surgical option were discussed and confirmed in a multidisciplinary tumour meeting. The decision to perform an augmentation procedure was based on FRL volumetry and/or functional evaluation based on Tc-99m Mebrofenin scintigraphy. In our center, liver growth was considered when expected FRL was <25–30% in normal liver, <35–40% in case of underlying liver disease (cirrhosis, cholestasis and/or prior chemotherapy), or Tc99m mebrofenin extraction below 2.69%/min/m2. The quality of the liver parenchyma was evaluated by liver biopsy in all patients.

If both parameters (volume and function) of the FRL were insufficient, or if liver scintigraphy was unavailable (Mebrofenin shortage), the radiologists decided to perform the LVD. By contrast, if a single parameter was abnormal, a simple PVE was performed.
Percutaneous embolization and patient management and operation were conducted by the same institution. Further evaluations of the FRL with contrast-enhanced computed tomography (CT) and Tc99m-Mebrofenin Scintigraphy were performed every week after PVE or LVD. Final surgical indication was based on both function and volume data.
Radiological procedure
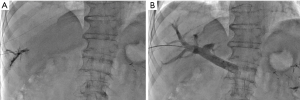
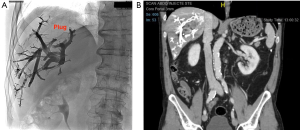
During LVD, right (and accessory right when present) hepatic vein was accessed under ultrasonographic guidance and a 0.018-inch microguidewire was inserted and left in place (Figure 2A). Then, PVE was performed using right transhepatic access (Figure 2B). After 3D portography, right portal vessels were embolized using a mixture of n-butyl cyanoacrylate and lipiodol (ratio 1:6). The microguidewire left in place in hepatic vein(s) was then used to insert a 7F-sheath in order to deploy an Amplatzer vascular plug II (75% oversizing). Finally, all distal venous branches were embolized using a mixture of n-butyl cyanoacrylate and lipiodol (ratio 1:6) (Figure 3).
Surgical procedure
Patients included in this study underwent a standard right hepatectomy (segments 5–8), according to the Brisbane classification of livers resection (ref). An intraoperative ultrasound was routinely performed to confirm the surgical resecability. The right hepatic artery and portal vein were systematically ligated and transected before the parenchymal transection with an anterior approach. Pringle manoeuver with intermittent clamping and right hepatic vein control were performed if necessary. The parenchymal phase was carried out with cavitron ultrasound aspirator (CUSA) or harmonic scalpel and bipolar forceps. At the end of the hepatic resection a haemostatic agent was used according to the surgeon decision.
Intra- and post-operative variables
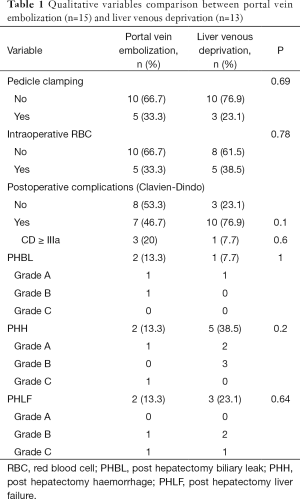
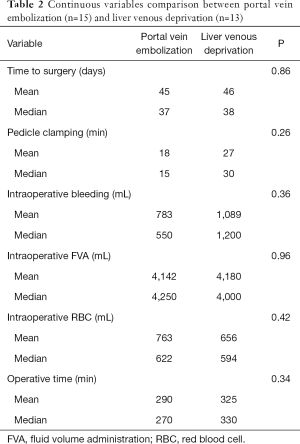
Intra- and post-operative data were recorded and compared between the two groups (Tables 1,2). Post-operative complications were graded according to Clavien-Dindo classification (21). PHLF, post-hepatectomy hemorrhage (PHH) and post-hepatectomy biliary leak (PHBL) were diagnosed and classified according to the criteria proposed by the International Study Group of Liver Surgery (ISGLS) (22-24). All the patients received the first visit after surgery one month after the discharge.
Full table
Full table
Pathological assessment
Surgical specimens were entirely evaluated according to the institutional protocols. Liver tissues were fixed in formalin (10%). Paraffin tissue sections were stained with hematoxylin and eosin or hematoxylin eosin and safran. A blind re-evaluation of all the specimens was made by the same pathologist. Liver samples of the embolized lobe were evaluated to assess histological changes of the resected specimen. Four parameters were compared between the two groups: atrophy, haemorrhage, necrosis and sinusoidal dilatation. A grade was assigned for evaluation of centro- and medio-lobular hepatic plates atrophy (none, slight to moderate or severe). Only severe grade was defined as positive in this study. Haemorrhagic areas (centro- and medio-lobular) were evaluated as present or absent. Necrosis (often focal, rarely diffuse, centro- and medio-lobular) was evaluated as present or absent. For the evaluation of dilatation of central veins, space of Disse and sinusoids a grade was also assigned (none, slight, moderate or severe). Only moderate and severe were defined as positive in this study.
Statistical analysis
Continuous data were expressed in median or mean and interquartile range (IQR) or range between minimum and maximum values, and compared using Student’s t-test or Mann-Whitney U test. For categorical data, the number and proportion (%) were displayed. Qualitative variables were compared by the Pearsons’s Chi square test or Fisher’s exact test when necessary. Statistical analyses were performed in SPSS 25.0 for Windows software (SPSS Inc., Chicago, Illinois, USA). P values were considered significant when less or equal than 0.05.
Results
To induce FRL growth, 16 patients underwent PVE and 13 underwent LVD. No complications related to the procedure were observed. Patients were resected for hepatocellular carcinoma (PVE n=9, LVD n=3), colorectal metastases (PVE n=5, LVD n=10), or others diseases (PVE n=2, LVD n=0). The patients with hepatocellular carcinoma (HCC) received trans-arterial chemoembolization (6 patients), radioembolization (3 patients), and NEXAVAR in 1 patient. No patient underwent to local ablative procedure prior to surgery. The patients with colorectal metastases received preoperative fluoropyrimidine-based chemotherapeutic regimens (FOLFIRI in 7 patients, FOLFOX in 4 patients, and XELOX in 1 patient), with the addition of biologic agents in 10 patients. Twelve patients received more than four cycles of chemotherapy before surgery (PVE n=5, LVD n=7).
Five patients were cirrhotic (PVE n=3, LVD n=2) with a Child-Pugh score graded as “A”. Surgery was confirmed for all the patients after the post embolization. The median time between radiologic embolization and operation was 37 days after PVE and 38 days after LVD (P=0.86). One patient of the PVE group had an intraoperative finding of carcinomatosis and thus liver resection was not performed. Surgical approach was open for the majority of patients (n=22), while 5 patients had a full laparoscopic hepatic resection (PVE n=3, LVD n=2).
Radiological outcomes
The overall baseline, FRL represented 31.2%±6.5% of the total liver volume whereas FRL was 40.8%±7.9% three weeks after liver preparation. Kinetic growth rate was 16±7 cc/day after LVD whereas it was 4.8±4 cc/day following PVE (P≤0.001).
Intra- and post-operative data analysis
Intraoperative necessity of pedicle clamping was similar in the two groups both in terms of number of cases (PVE n=5, LVD n=3, P=0.69) and total duration (median: PVE =15 min, LVD =30 min, P=0.26). No significant difference was found when comparing intraoperative bleeding (median: PVE =550 mL, LVD =1,200 mL, P=0.36) and fluid volume administration (median: PVE =4,250 mL, LVD =4,000 mL, P=0.96). As regards intraoperative transfusions, 5 patients received red blood cells (RBC) during surgery in both groups (P=0.78), without any difference in terms of total volume of RBC transfused (median: PVE =622 mL, LVD =594 mL, P=0.42). Mean operative time in PVE group was 290 min (range, 150–598 min) and 325 min in patients undergoing LVD (range, 177–428 min). No statistical difference was found between the two groups (P=0.34). Post-operative course was similar when comparing both medical and surgical complications in the two arms (PVE n=7, LVD n=10, P=0.1). Major complications (Clavien-Dindo ≥ IIIa) occurred in 3 patients undergoing PVE (20%) and in 1 patient (7.7%) of the LVD group (P=0.6). Among these complications, two were classified IIIa (radiologic or endoscopic procedure) and one V, a patient (PVE group) died for an acute PHLF secondary to a left portal vein thrombosis. PHBL rate was similar (PVE n=2, LVD n=1, P=1), all cases classified as B according to ISGLS grading system. No significant difference was found when comparing the appearance of PHLF (PVE n=2, LVD n=3, P=0.64) between PVE and LVD. Two patients received RBC after surgery in the PVE group whereas 5 patients were transfused in the LVD group (P=0.2). Only in one case reoperation was necessary (PHH grade C according to ISGLS, PVE group), secondary to wound bleeding in the patient later deceased for PHLF.
Histological assessment
A major hepatocyte atrophy in the embolized lobe was seen in five patients after LVD and in two patients after PVE (P=0.19). Hemorrhage and necrosis were slightly more frequent in the LVD group, without statistical relevance (PVE n=4, LVD n=6, P=0.42; PVE n=4, LVD n=5, P=0.5). Concerning sinusoidal dilatation, six and seven patients presented an important distension of central veins, space of Disse and sinusoids respectively after PVE and LVD (P=0.69).
Discussion
LVD has been recently proposed as an alternative technique to induce FRL hypertrophy, in order to decrease the risk of PHLF after major hepatectomies (12). It consists of simultaneous embolization of one or two major hepatic veins and their tributaries as well as of the portal vessels of the future resected liver resulting in venous deprivation. This idea derives from the demonstration that hepatic outflow occlusion some weeks after PVE, in patients who had shown limited hepatic regeneration, was safe and could further increase contralateral liver volume (13-16). In 2009 Hwang et al. (14) in fact reported in his series a further rise of the DH at 28.9% after secondary hepatic vein embolization (HVE) compared to 13.3% reached after PVE, allowing more patients to undergo surgical treatment. However, this sequential approach does not spare time compared to PVE alone and, more importantly, the greater DH noted in the HVE + PVE group from the Hwang series was seen in the post-HVE FLR evaluation. This is the reason why the combination of both HVE and PVE during the same procedure was assessed with promising results reported (12,18,19). We must acknowledge technical variations among these reported series. The term “bi-embolization” refers to plug deployment in a major hepatic vein without any additional embolization of venous tributaries (19). Veno-venous collaterals are often present in normal livers, and can enlarge after HV occlusion (25). When performing LVD through a transhepatic access, we frequently see these collaterals enlarging within several minutes after plug deployment (12-18) owing to iodine contrast medium injection behind the plug. These collaterals can circumvent the plug with high flow and make ‘bi-embolization’ useless, explaining why we rather perform LVD by embolizing distal venous branches (and veno-venous shunts) using glue. In addition, contrary to LVD, bi-embolization promotes veno-venous shunts creation and enlargement and could make surgical resection more difficult secondary to an increased intra-operative bleeding. The need for exploring new procedures to achieve a better and faster DH derives from some drawbacks shown by the classic PVE and the ALPPS technique. The first, despite its big success, needs up to 6 weeks before a safe surgery and it is not always associated with a sufficient DH. Indeed, about 20% of the patients will not be able to undergo surgical resection due to the risk of tumor progression or a low FRL hypertrophy (4). On the other hand, ALPPS has encountered several pitfalls and it is still a controversial procedure. Since its description (26), it has been considered as a revolution in liver surgery for its extraordinary capacity of modifying the FRL. The first series surprisingly showed a median volumetric increase up to 70–80% in about half the time (26,27) with a consequently higher rate of completing the surgical stage. In our series, the kinetic liver growth rate was significant higher in the LVD group compared to the PVE group. These results are compatible with an earlier timing of the operation for the LVD group. In spite of that, we were not able to schedule patients earlier due to administrative constraints which explains why intervals to surgery are similar in the two groups.
Nevertheless, our results have been strongly opposed by the analysis of the morbidity and mortality rate in patients undergoing ALPPS. Several meta-analyses reported a higher risk of post-operative complications compared to conventional two-stage hepatectomy (9-11), due probably to a less functioning FRL (28,29). More strategies should therefore be investigated. Preliminary data on LVD procedure assessed a good feasibility and a comparable tolerance to simple PVE (12,18,19,30). Despite the limited number of patients, in our series no complication occurred during and after LVD and all the patients could undergo surgical resection. Simultaneous portal and hepatic vein embolization were well tolerated even in those patients with a fragile liver function secondary to cirrhosis or prior chemotherapy. To the best of our knowledge, only one study in literature compared LVD to standard PVE in terms of surgical complications (30). However, in this series only twelve patients with a Klatskin tumor undergoing PVE + Biliary drainage or LVD + biliary drainage before surgery were analyzed. Only few pre-, intra-, and post-operative data were available for evaluation with a weak statistical power for this limited study population. In our cohort several pre-, intra-, and post-operative variables were assessed and compared between the two groups, and no statistical difference was found. The importance of a perioperative assessment in these patients arises from some findings described after LVD. The increased FRL hypertrophy after the occlusion of the hepatic outflow could in fact be partially explained by changes in portal pressure and hepatic flow (17). Mohkam et al. (31) recently described that hepatic venous pressure gradient after PVE is an important benchmark to predict accurately FRL hypertrophy. This theory is corroborated by sinusoid dilatation on the pathological specimen and the immediate creation of veno-venous shunts during the procedure described by our team (12). In our series all the histological findings (hepatocyte atrophy, hemorrhage, necrosis, sinusoid dilation, distension of central veins, space of Disse and sinusoids) of the embolized liver parenchyma assessed were more marked after LVD although no statistical difference was observed. Interestingly, a previous study showed that about 75% of patients presents these veno-venous shunts after right hepatic vein occlusion, and they are often undiagnosed in the pre-procedural CT scan evaluation (30). Nevertheless, these hemodynamic variations after LVD could hypothetically determine a vascular congestion and increase in the risk of intraoperative bleeding and post-operative hemorrhage. In the current experience similar data were found when comparing necessity of pedicle clamping during the parenchymal phase, intraoperative bleeding and transfusions. In the post-operative course PHH incidence was higher in the LVD group, though not statistically significant. Although a safe FRL is achieved before surgery, PHLF remains an important complication in patients who benefited from a two-stage hepatectomy. A recent review emphasized this data in patients undergoing ALPPS (32). Indeed, the incidence of PHLF after this procedure stands, on average, at 30% and up to 75% of all mortalities after surgical stage are likely a consequence of PHLF. In our cohort no additional risk of PHLF was found after LVD.
Our study displays several strengths. This is the largest series described in literature which compares intra- and post-operative outcomes after LVD and PVE in patients underwent right hepatectomy. A strict selection of inclusion and exclusion criteria limited our simple size but increased the homogeneity and the power of these results. In order to focus on the perioperative impact of the LVD, a large number of intra- and post-operative variables were considered and compared. At our knowledge, there is no other study in literature comparing the surgical (intra-, per-, post-operative) outcomes of the two techniques. Lastly, cirrhotic patients with no clinical or biochemical impairment (Child A) were included and assessed in both groups. Although no statistical analysis was possible for the limited number of cases, no complications occurred in these patients after LVD and the FRL hypertrophy was sufficient to undergo a safe surgical resection.
The present study presents also several limitations. Due to the recent nature of this technique, few consecutive patients were included in this study and they presented with a heterogenous subset of liver conditions. The study is retrospective although data was prospectively collected. Randomized controlled trials (RCT) are needed to confirm the benefit of LVD. Actually, one RCT (promoted by our team) started in France, and another International RCT called “DRAGON1” is working in progress.
In conclusion, the LVD technique is feasible, well tolerated and provides fast and important hypertrophy of the FRL, without influencing the morbidity/mortality rate during and after right hepatectomy.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. An informed consent was acquired before both the embolization and the surgical operation. This study was approved by the Institutional Review Board (#2019-68) and it was aligned to Helsinki declaration.
References
- Schreckenbach T, Liese J, Bechstein WO, et al. Posthepatectomy liver failure. Dig Surg 2012;29:79-85. [Crossref] [PubMed]
- Huang SY, Aloia TA. Portal vein embolization: State-of-the-Art Technique and Options to Improve Liver Hypertrofy. Visc Med 2017;33:419-25. [Crossref] [PubMed]
- Makuuchi M, Thai BL, Takayasu K, et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery 1990;107:521-7. [PubMed]
- van Lienden KP, van den Esschert JW, de Graaf W, et al. Portal vein embolization before liver resection a systematic review. Cardiovasc Intervent Radiol 2013;36:25-34. [Crossref] [PubMed]
- Yamashita S, Sakamoto Y, Yamamoto S, et al. Efficacity of preoperative portal vein embolization among patients with hepatocellular carcinoma, biliary tract cancer and colorectal liver metastases: a comparative study based on single center experience of 319 cases. Ann Surg Oncol 2017;24:1557-68. [Crossref] [PubMed]
- Ribero D, Abdalla EK, Madoff DC, et al. Portal vein embolization before major hepatectomy and its effects on regeneration, resectability and outcome. Br J Surg 2007;94:1386-94. [Crossref] [PubMed]
- Isfordink CJ, Samim M, Braat MNGJA, et al. Portal vein ligation versus portal vein embolization for induction of hypertrophy of the future liver remnant: A systematic review and meta-analysis. Surg Oncol 2017;26:257-67. [Crossref] [PubMed]
- Pandanaboyana S, Bell R, Hidalgo E, et al. A systematic review and meta-analysis of portal vein ligation versus portal vein embolization for elective liver resection. Surgery 2015;157:690-8. [Crossref] [PubMed]
- Eshmuminov D, Raptis DA, Linecker M, et al. Meta-analysis of associating liver partition with portal vein ligation and portal vein occlusion for two-stage hepatectomy. Br J Surg 2016;103:1768-82. [Crossref] [PubMed]
- Tustumi F, Emani L, Coelho FF, et al. Preoperative strategies to improve resectability for hepatocellular carcinoma: a systematic review and meta-analysis. HPB (Oxford) 2018;20:1109-18. [Crossref] [PubMed]
- Zhou Z, Xu M, Lin N, et al. Associating liver partition and portal vein ligation for staged hepatectomy versus conventional two-stage hepatectomy: a systematic review and meta-analysis. World J Surg Oncol 2017;15:227. [Crossref] [PubMed]
- Guiu B, Chevallier P, Denys A, et al. Simultaneous trans hepatic portal and hepatic vein embolization before major hepatectomy the liver venous deprivation technique. Eur Radiol 2016;26:4259-67. [Crossref] [PubMed]
- Hwang S. Sequential preoperative ipsilateral hepatic vein embolization after portal vein embolization to induce further liver regeneration in patients with hepatobiliary malignancy. Ann Surg 2009;249:608-16. [Crossref] [PubMed]
- Hwang S, Lee SG, Ko GY, et al. Preoperative sequential portal and hepatic vein embolization in patients with hepatobiliary malignancy. World J Surg 2015;39:2990-8. [Crossref] [PubMed]
- Ko GY, Hwang S, Sung KB, et al. Interventional oncology: new options for interstitial treatments and intravascular approaches: right hepatic vein embolization after right portal vein embolization for inducing hypertrophy of the future liver remnant. J Hepatobiliary Pancreat Sci 2010;17:410-2. [Crossref] [PubMed]
- Munene G, Parker RD, Larrigan J, et al. Sequential preoperative hepatic vein embolization after portal vein embolization for extended left hepatectomy in colorectal liver metastases. World J Surg Oncol 2013;11:134. [Crossref] [PubMed]
- Schadde E, Guiu B, Deal R, et al. Simultaneous hepatic and portal vein ligation induces rapid liver hypertrophy: A study in pigs. Surgery 2019;165:525-33. [Crossref] [PubMed]
- Guiu B, Quenet F, Escal L, et al. Extended liver venous deprivation before major hepatectomy induces marked and very rapid increase in future liver remnant function. Eur Radiol 2017;27:3343-52. [Crossref] [PubMed]
- Le Roy B, Perrey A, Fontarensky M, et al. Combined preoperative portal and hepatic vein embolization (biembolization) to improve liver regeneration before major liver resection: a preliminary report. World J Surg 2017;41:1848-56. [Crossref] [PubMed]
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453-7. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Rahbari NN, Garden OJ, Padbury R, et al. Posthepatectomy liver failure: A definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011;149:713-24. [Crossref] [PubMed]
- Rahbari NN, Garder OJ, Padbury R, et al. Post-hepatectomy haemorrhage: a definition and grading by the International Study Group of Liver Surgery (ISGLS). HPB (Oxford) 2011;13:528-35. [Crossref] [PubMed]
- Koch M, Garden OJ, Padbury R, et al. Bile leakage after hepatobiliary and pancreatic surgery: A definition and grading of severity by the International Study Group of Liver Surgery. Surgery 2011;149:680-8. [Crossref] [PubMed]
- Sakaguchi T, Suzuki S, Inaba K, et al. Analysis of intrahepatic venovenous shunt by hepatic venography. Surgery 2010;147:805-10. [Crossref] [PubMed]
- Schnitzbauer AA, Lang SA, Goessmann H, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg 2012;255:405-14. [Crossref] [PubMed]
- Schadde E, Ardiles V, Slankamenac K, et al. ALPPS offers a better chance of complete resection in patients with primarily unresectable liver tumors compared with conventional-staged hepatectomies: results of a multicenter analysis. World J Surg 2014;38:1510-9. [Crossref] [PubMed]
- Olthof PB, Tomassini F, Huespe PE, et al. Hepatobiliary scintigraphy to evaluate liver function in ALPPS: liver volume overestimates liver function. Surgery 2017;162:775-83. [Crossref] [PubMed]
- Truant S, El Amrani M, Skrzypczyk C, et al. Factors associated with fatal liver failure after extended hepatectomy. HPB (Oxford) 2017;19:682-7. [Crossref] [PubMed]
- Hocquelet A, Sotiriadis C, Duran R, et al. Preoperative portal vein embolization alone with biliary drainage compared to combination of simultaneous portal vein, right hepatic vein embolization and biliary drainage in Klatskin tumor. Cardiovasc Intervent Radiol 2018;41:1885-91. [Crossref] [PubMed]
- Mohkam K, Rode A, Darnis B, et al. Hepatic venous pressure gradient after portal vein embolization: An accurate predictor of future liver remnant hypertrophy. Surgery 2018;164:227-32. [Crossref] [PubMed]
- Kang D, Schadde E. Hypertrophy and Liver Function in ALPPS: Correlation with Morbidity and Mortality. Visc Med 2017;33:426-33. [Crossref] [PubMed]


