
Left hepatectomy with caudate lobe resection using the methylene blue staining technique for bismuth IIIb hilar cholangiocarcinoma
Left hepatectomy with caudate lobe resection has been widely accepted as the standard treatment for bismuth IIIb hilar cholangiocarcinoma (1-6). However, there is no clear boundary between the right caudate lobe and the right posterior section. The methylene blue staining technique are commonly used to delineate the margin of the relevant hepatic segment for anatomic hepatectomy (7). Herein, we described this technique to demarcate the boundary between the caudate lobe and the right posterior lobe for an en bloc resection of the tumor and improve the R0 surgical margin.
A reversal L shape incision (upper median plus right-sided sub costal transverse incision) is performed in a supine position.
After Kocher’s mobilization, we perform skeletonization of the hepatoduodenal ligament. The common and proper hepatic arteries are separated, and then the left and right hepatic artery is exposed, the left hepatic artery is doubly ligated and divided at the root. The common bile duct is ligated and divided near the upper edge of the pancreatic head and frozen section is performed. The stump of the common bile duct is pulled in the cranio-ventral direction, and skeletonization of the hepatic artery and portal vein extends upward to the hepatic hilum (Figure 1A).
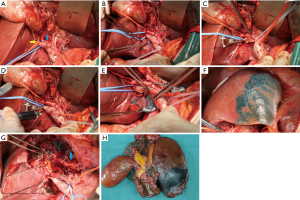
The portal trunk and portal vein bifurcation are sequentially skeletonized and the right portal vein should be exposed as far as possible. There are two or three tiny caudate lobe portal branches behind the right and left portal veins and junctions (Figure 1B). Carefully separation these branches. The rubber band tube suspends the right portal vein, temporarily blocking by the bulldog clamp (Figure 1C), and then inject the methylene blue through the portal vein trunk (Figure 1D). At this point, the border of the left hemi-liver and caudate lobe appears on the surface of the liver (Figure 1E,F). The left portal vein and the tiny caudate lobe portal branches are ligated and divided at the root.
The right and left hemi-livers are fully mobilized from their attachments. Arantius ligament is then isolated, ligated, and divided. The short hepatic veins from the caudate lobe to the inferior vena cava are divided and the roots of the three hepatic veins are exposed.
There is already a boundary between the liver surface and the liver parenchyma. Liver resection usually begins at the edge of the surface boundary and proceeds according to the staining range in the liver parenchyma. After the middle hepatic vein (MHV) is found in parenchyma, the MHV should be fully exposed and care should be taken. When the MHV is completely exposed to its root, the left hepatic vein is encircled and dissected. After complete exposure of the dorsal surface of the MHV, the direction of hepatectomy must be changed again toward the right edge of the inferior vena cava (IVC). Since we have performed left hepatic and caudate lobe staining, we can find a suitable resection plane between the right caudate lobe and segment 7. The liver parenchyma is separated along this staining boundary and the right hepatic vein (RHV) is completely exposed. When the liver resection is completed, the left and right livers are only connected through the portal connective tissue and contains the right hepatic duct. At this point, the bile duct is sectioned and sent for frozen analysis (Figure 1G,H).
Roux-en-Y biliary anastomosis is finally performed with 5-0 or 6-0 absorbable suture threads. If frozen section is positive, continue to remove the bile duct upward.
In our center, we have completed 22 cases using this technique from January 2014 to November 2018. R0 resection was achieved in 17 of 22 patients, and no serious complications.
Left hepatectomy is used to treat hilar cholangiocarcinoma mainly involving the left side of the hepatic hilum (5). However, some authors (6) have reported that the positive margin of tumor in patients with left hepatectomy was significantly higher than that of the right hepatectomy. The reason may be that the extrahepatic part of the right hepatic duct (the remanent side after left hepatectomy) is significantly shorter, farther from the hepatic bifurcation, and the branch of the right liver is also much shorter than the left liver (5,6). In addition, in most patients, the right posterior bile duct runs cranially around the right portal vein to merge with the right anterior bile duct. For this right posterior bile duct confluence, it is more difficult to obtain a negative margin (8,9). Because of these anatomical variations, the complete right caudate lobe resection can fully reveal the right hepatic duct, especially the right posterior hepatic duct, and free it to the upper branch, so as to achieve the negative bile duct margin.
However, in the case of complete caudate lobe resection, there is no clear boundary between the right caudate lobe and the right posterior section. According to the anatomy of the caudate lobe, 2 to 5 small branches from the left branch, the right branch, and/or the bifurcation of the portal vein are grouped into the caudate lobe (10). Since these caudate lobe branches are located on the dorsal side and are small and scattered, it is difficult to inject methylene blue into these small portal veins. Therefore, we block the right portal veins and inject methylene blue through the portal vein trunk, so that the left hemi-hepatic and the whole caudate lobe can be stained. Then we ligated the left branch of the portal vein and the pedicles of the caudate lobe to avoid the dye from excretion with the portal blood flow, achieving the effect of long-lasting staining. The range of staining in the parenchyma identifies the resection plane, which improves the accuracy of total caudate lobe resection.
In our experiences, methylene blue staining technology can be a useful tool for an en bloc resection of the tumor and improve the R0 surgical margin.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Hemming AW, Reed AI, Fujita S, et al. Surgical management of hilar cholangiocarcinoma. Ann Surg 2005;241:693-9; discussion 699-702. [Crossref] [PubMed]
- Igami T, Nishio H, Ebata T, et al. Surgical treatment of hilar cholangiocarcinoma in the "new era": the Nagoya University experience. J Hepatobiliary Pancreat Sci 2010;17:449-54. [Crossref] [PubMed]
- Unno M, Katayose Y, Rikiyama T, et al. Major hepatectomy for perihilar cholangiocarcinoma. J Hepatobiliary Pancreat Sci 2010;17:463-9. [Crossref] [PubMed]
- Uesaka K. Left hepatectomy or left trisectionectomy with resection of the caudate lobe and extrahepatic bile duct for hilar cholangiocarcinoma (with video). J Hepatobiliary Pancreat Sci 2012;19:195-202. [Crossref] [PubMed]
- Shimizu H, Kimura F, Yoshidome H, et al. Aggressive surgical resection for hilar cholangiocarcinoma of the left-side predominance: radicality and safety of left-sided hepatectomy. Ann Surg 2010;251:281-6. [Crossref] [PubMed]
- Konstadoulakis MM, Roayaie S, Gomatos IP, et al. Aggressive surgical resection for hilar cholangiocarcinoma: is it justified? Audit of a single center's experience. Am J Surg 2008;196:160-9. [Crossref] [PubMed]
- Shou-wang C, Shi-zhong Y, Wen-ping L, et al. Sustained methylene blue staining to guide anatomic hepatectomy for hepatocellular carcinoma: Initial experience and technical details. Surgery 2015;158:121-7. [Crossref] [PubMed]
- Ohkubo M, Nagino M, Kamiya J, et al. Surgical anatomy of the bile ducts at the hepatic hilum as applied to living donor liver transplantation. Ann Surg 2004;239:82-6. [Crossref] [PubMed]
- Shimizu H, Sawada S, Kimura F, et al. Clinical significance of biliary vascular anatomy of the right liver for hilar cholangiocarcinoma applied to left hemihepatectomy. Ann Surg 2009;249:435-9. [Crossref] [PubMed]
- Benkö T, Sgourakis G, Molmenti EP, et al. Portal Supply and Venous Drainage of the Caudate Lobe in the Healthy Human Liver: Virtual Three-Dimensional Computed Tomography Volume Study. World J Surg 2017;41:817-24. [Crossref] [PubMed]


