
A prognostic nomogram for patients with resected fibrolamellar hepatocellular carcinoma
Introduction
Hepatocelluar carcinoma (HCC) is the most common primary liver cancer. In 2018, 42,200 new cases of primary liver cancer are anticipated in the United States, with 30,200 related deaths, making it the 10th most common cancer in males and 5th highest cause of cancer deaths (1). Fibrolamellar hepatocellular carcinoma (FLHC) is a variant of HCC. It comprises approximately 0.85% of all primary liver tumors in the United States and between 1% and 9% of all HCCs (2,3). It is distinct from conventional HCC as it affects younger patients, from 10 to 35 years old, without underlying liver dysfunction compared to the average 65 years old with liver disease is diagnosed with conventional HCC (2). While the etiology of FLHC is unclear, it is thought to have a more indolent course and an overall better prognosis than HCC and other primary liver tumors (2,4-6).
When feasible, surgical resection with negative margins represents the only potential curative option. Five year survival of those who undergo resection can reach greater than 70%, while those who do not undergo surgery have significantly worse outcomes with a 100% mortality at 5 years (7-9). Traditionally, FLHC has been thought to have a more indolent course and better prognosis than conventional HCC. However, data from MD Anderson reveals similar 5-year and median survival in patients after resection with FLHC compared to non-cirrhotic HCC patients (2). Recurrence rates following resection of FLHC range from 33–100% with median recurrence-free survival rates of 20–48 months (4,10). The historically noted higher overall survival may be in part due to the younger age at diagnosis and the absence of cirrhosis.
Despite the differences noted in FLHC and conventional HCC, they are included in the same American Joint Committee on Cancer (AJCC) staging classification (11). However, studies have found that the AJCC staging classification for HCC does not stratify recurrence-free survival in FLHC accurately (8). Several studies have investigated potential prognostic factors in FLHC, most being single institution analysis. Factors associated with better prognosis following surgery including older age at diagnosis, earlier tumor stage at diagnosis, fewer tumors at diagnosis, R0 resection, normal liver enzymes, as well as absence of invasion of large vessels or thrombosis (3,8). However, most of these series are single institution small retrospective analyses. The aim of this study was to examine post-resection outcomes and prognostic indicators for survival in a large national cohort of patients from the National Cancer Database (NCDB).
Methods
Data source and study population
This study was a retrospective analysis of prospectively collected data from the NCDB database. The NCDB is a jointly sponsored database by the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The (NCDB), established in 1989, derives data from more than 1,500 CoC accredited programs capturing more than 70% of newly diagnosed cancer cases nationally. These data include patient demographics, tumor characteristics, treatment course including surgical and medical therapies, surveillance, quality measures, complications and survival. The CoC designates cancer programs based on ability to provide a wide range of oncological services and specialists. CoC-approved hospitals are larger, perform more operations and provide more cancer-related services to patients than non-CoC hospitals (12).
The NCDB shared files contain site-specific de-identified data on more than 80 variables comprising sociodemographic, tumor, treatment and follow-up information. These data are abstracted by certified tumor registrars from medical records, even if the care extends to a non-CoC facility. The NCDB does not specify the frequency of follow-up, but sets the standard of 90 per cent at 5 years. Quality is assured by the CoC by means of more than 600 electronic automated checks to maximize internal consistency and minimize missing data. In addition, the CoC also performs routine audits to ensure data quality and completeness (13). Institutional review board approval was not required for this study as patient de-identified data were analyzed.
Patients with a diagnosis of FLHC who underwent resection from 2004 to 2014 within the NCDB database were identified by the International Classification of Diseases for Oncology, Third Edition (ICD-O-3) topography code for the site of origin and histology. Only patients with who underwent a cancer-directed curative intent operation with confirmed histology were included. Patients were excluded if they were not histologically confirmed, had multiple primary cancers, metastatic disease, or if lymph node status was unknown.
Outcomes and predictive variables
Clinicopathologic information including patient age, sex, race, year of resection, grade, tumor size, lymph node status, distant metastases, adjuvant chemotherapy, adjuvant radiation, time to chemotherapy, margin status, academic vs. non-academic facility were collected from the NCDB database. Operations were classified as ablative procedures only (including radiofrequency ablation or microwave ablation), hepatic resection or liver transplantation, and surgery not otherwise specified. The types of hepatic resections when available were divided into three groups: partial and hemihepatectomy, extended hepatectomy, and liver transplantation. Variables were transformed into categorical and indicator variables when appropriate. Only patients who underwent a hepatic resection were included in the analysis.
Statistical analyses
All statistical analyses were performed using STATA software, version 14.2 (StataCorp LLC, College Station, TX, USA). Continuous variables were noted as median values with standard deviations and discrete values were noted as frequencies. Overall survival was calculated using the date of diagnosis of FLHC and date of last contact or death. Univariate comparisons were performed using the two-tailed Student’s t-test for these continuous variables. Categorical variables were analyzed using Pearson chi-square (χ2) tests. Cox proportional hazard model was performed for univariate as well as multivariate models. Covariates were included in the multivariate model according to statistical significance in univariate analysis. Relative risks were reported as hazard ratios (HR) with a 95% confidence interval (CI). P<0.05 was considered statistically significant for all tests.
A nomogram was developed using STATA software, version 14.2 (StataCorp LLC, College Station, TX, USA). Multivariate analysis of predictors of survival was performed using stepwise Cox regression with backward elimination with a P value threshold of 0.1. To validate the nomogram, discrimination was quantified using Harrell’s c concordance index (c-index) as well as Somers’ D as a natural extension of the area under the receiver operating characteristic (ROC) area to survival analysis. Kaplan-Meier analysis was used to calculate cumulative event rates and survival curves were compared using log-rank test for stages 1, 2, 3 and 4 according to the AJCC staging system. Overall survival was calculated using the date of diagnosis of FLHC and date of last contact or death.
Results
Patient and tumor characteristics
The NCDB query yielded 461 patients diagnosed with FLHC between 2004 and 2014. After excluding patients with no histologic verification of FLHC, unknown lymph node status, known metastatic disease and those who did not undergo a cancer directed operation, 197 patients remained. Moreover, only patients with long term follow up data were included leaving 171 (86.8%) patients available for analysis.
The demographic and clinical characteristics of the 171 patients who underwent resection for FLHC are outlined in Table 1. The median age for patients with FLHC was 34.0 (IQR, 22–39) years. Half of the patients were female (n=87, 50.9%). A majority of the patients were Caucasian (n=137, 80.1%). The median tumor size was 9.2 (IQR, 6.2–13.1) cm, and 22% of patients had regional node-positive disease on final pathology. Vascular invasion, which was defined as invasion of the branches of the main portal vein (right or left portal vein, not including sectoral or segmental branches) or as invasion of 1 or more of the 3 hepatic veins (right, middle, or left), was seen in 44 (25.7%) tumors. A majority of patients had an unknown tumor grade (77, 45.0%) while 23 (13.5%) were low grade, 66 (38.6%) were intermediate and only 12 (7.0%) were high grade.
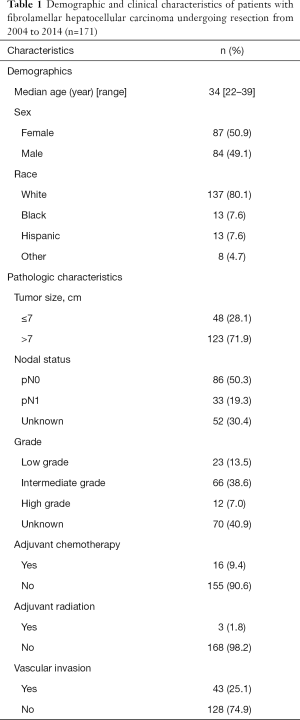
Full table
Long term outcomes
Median follow up time was 50 months (IQR, 24–78 months). The overall median survival of patients with FLHC who underwent resection was 87.95 (IQR, 36.67–134.3) months. On univariate analysis, several factors were associated with decreased overall survival including age, tumor size >7 cm, multifocal disease, adjuvant chemotherapy, lymph node positive disease on final pathology, and vascular invasion (all P<0.05; Table 2). Type of surgical resection was not associated with overall survival. After using multivariate analysis to control for clinicopathologic risk factors, age (HR 1.03; 95% CI: 1.01–1.05; P=0.003), vascular invasion (HR 1.75; 95% CI: 1.00–3.09; P=0.050), tumor size >7 cm (HR 2.18; 95% CI: 1.02–4.66; P=0.044), multifocal disease (HR 3.34; 95% CI: 1.53–7.25; P=0.002), and lymph node positive disease on final pathology (HR 2.75; 95% CI: 1.42–5.35; P=0.003) all remained independent negative predictors of overall survival (Table 2).
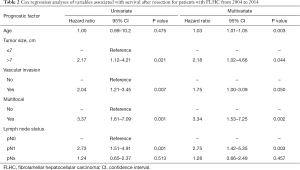
Full table
Prognostic nomogram
A prognostic nomogram was generated by integrating all of the independent factors for overall survival into the newly constructed model (Figure 1). The calibration curve and C-index were used to assess the discriminant ability of the nomogram. The C-index for the nomogram was 0.710, whereas, the C-index for this cohort using the AJCC staging was 0.654. Thus, this nomogram was a better predictor of survival than the AJCC stage.
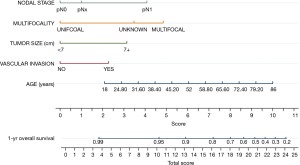
Discussion
In this study, independent risk factors for poor overall survival in patients with FLHC who underwent resection were identified and used to create a novel and improved nomogram for predicting survival after curative intent resection. The C-index is a method to assess the degree of conformity between prediction and actual outcome. The discriminant ability of our nomogram was superior to that of the AJCC staging (0.710 vs. 0.654) which is currently the most widely-used system for the classification and prognosis of FLHC. This nomogram integrates several individual clinical and pathologic variables and provides an individualized risk assessment for patients.
Our results indicated that the nomogram we created has a higher prediction accuracy for overall survival than the AJCC staging system (0.710 vs. 0.654). The final nomogram incorporated five independent risk factors for OS including age, vascular infiltration, tumor size >7 cm, multifocality, lymph node positivity. Previous studies have suggested improved prognosis for patients with FLHC following surgery with older age at diagnosis, earlier tumor stage at diagnosis, fewer tumors at diagnosis, R0 resection, normal liver enzymes, and the absence of invasion of large vessels or thrombosis (3,8).
Currently, surgery with negative margins represents the only option for a cure in FLHC. However, recurrence rates following resection of FLHC range from 33–100% and median recurrence-free survival rates of 20–48 months (4,10). Adjuvant and neoadjuvant treatments have not proved effective in FLHC. Multiple studies have shown that lymph node positivity rates are higher in FLHC than in traditional hepatocellular carcinoma (14-16). McAteer et al. showed that five year survival for lymphadenectomy patients was superior to non-lymphadenectomy patients (70% vs. 53%) and adjusted mortality for lymphadenectomy patients was superior to non-lymphadenectomy patients (14,15). Therefore, periportal lymphadenectomy should be performed when resecting FLHC.
Recently, a novel fusion gene, DNAJB1-PRKACA was identified in tumor samples from 11 patients by who genome sequencing but not in any of the matched healthy liver tissue (17). This finding has been confirmed by multiple independent studies (18-21). Thus, DNAJB1-PRKACA may serve as a tissue biomarker for FLHC and if secreted into circulation may actually be useful as a plasma biomarker. As no other genetic aberrations have been identified in FLHC, DNAJBI-PRKACA is likely involved early malignant transformation, making it a candidate to be an effective therapeutic target.
Ultimately, the discriminant ability of the nomogram may be even further refined in the future to include additional predictors. Potential biomarkers, such as DNAJBI-PRKACA may improve patient selection and better prognosticate who would benefit from neoadjuvant or adjuvant therapies especially as more effective targeted therapies are identified.
At this time, both National Comprehensive Cancer Network (NCCN) guidelines and AJCC staging do not discriminate between classic HCC and FLHC. Thus, determining patient prognosis as well as risk of recurrence after surgery remains difficult. The current study identified age, vascular infiltration, tumor size >7 cm, multifocality, lymph node positivity as independent factors in survival thus creating a risk profile to better inform patients, identify those at high risk of recurrence and ultimately, who may benefit from adjuvant treatments as they become available. Patients identified to be high risk using this nomogram post operatively should undergo strict imaging surveillance. Currently NCCN guidelines recommend imaging every 3–6 months for 2 years, followed by every 6–12 months following resection for HCC. Therefore, these high risk patients should undergo surveillance every 3 months for 2 years, followed by every 6 months. As these patients tend to be significantly younger, the duration of surveillance has yet to be determined.
Although our nomogram performed better than that of the AJCC staging, there are limitations. Our results are from a population within the United States and thus may not be translated to other areas of the world. The variables are dependent on pathology from surgery, therefore, it is most useful in the post-operative setting. Ideally, to reduce the risk of overfitting, a bootstrap resampling method would be used, however, the specific variables studied are not recorded stringently in other databases available at this time. Long term prognosis for greater than 5 years is unable to be calculated with this dataset, but will need to ultimately be looked at using this nomogram in future studies. In addition, as our nomogram is derived from data retrospectively collected and designed to predict future survival, the results should be confirmed in a prospective cohort study.
Conclusions
In conclusion, this study demonstrates a novel nomogram for patients with resected FLHC. This nomogram predicts improved OS which includes age, tumor size, multifocality, vascular infiltration and lymph node positivity. External validation and expansion of this nomogram as new biomarkers are identified are necessary.
Acknowledgments
Funding: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award number NIH 5K12CA001727-20. The content is solely the responsibility of Laleh Melstrom and does not necessarily represent the official views of the National Institutes of Health.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Due to the deidentified nature of this data set, this study was exempt from institutional review board approval.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- El-Serag HB, Davila JA. Is fibrolamellar carcinoma different from hepatocellular carcinoma? A US population-based study. Hepatology 2004;39:798-803. [Crossref] [PubMed]
- Moreno-Luna LE, Arrieta O, Garcia-Leiva J, et al. Clinical and pathologic factors associated with survival in young adult patients with fibrolamellar hepatocarcinoma. BMC Cancer 2005;5:142. [Crossref] [PubMed]
- Stipa F, Yoon SS, Liau KH, et al. Outcome of patients with fibrolamellar hepatocellular carcinoma. Cancer 2006;106:1331-8. [Crossref] [PubMed]
- Craig JR, Peters RL, Edmondson HA, et al. Fibrolamellar carcinoma of the liver: a tumor of adolescents and young adults with distinctive clinico-pathologic features. Cancer 1980;46:372-9. [Crossref] [PubMed]
- Ang CS, Kelley RK, Choti MA, et al. Clinicopathologic characteristics and survival outcomes of patients with fibrolamellar carcinoma: data from the fibrolamellar carcinoma consortium. Gastrointest Cancer Res 2013;6:3-9. [PubMed]
- Mavros MN, Mayo SC, Hyder O, et al. A systematic review: treatment and prognosis of patients with fibrolamellar hepatocellular carcinoma. J Am Coll Surg 2012;215:820-30. [Crossref] [PubMed]
- Yamashita S, Vauthey JN, Kaseb AO, et al. Prognosis of Fibrolamellar Carcinoma Compared to Non-cirrhotic Conventional Hepatocellular Carcinoma. J Gastrointest Surg 2016;20:1725-31. [Crossref] [PubMed]
- Chun YS, Zimmitti G. Fibrolamellar variant of hepatocellular carcinoma. Recent Results Cancer Res 2013;190:101-10. [Crossref] [PubMed]
- Groeschl RT, Miura JT, Wong RK, et al. Multi-institutional analysis of recurrence and survival after hepatectomy for fibrolamellar carcinoma. J Surg Oncol 2014;110:412-5. [Crossref] [PubMed]
- Amin MB, American Joint Committee on Cancer, American Cancer Society. AJCC cancer staging manual. Eighth edition. Springer International Publishing, 2017.
- Bilimoria KY, Bentrem DJ, Stewart AK, et al. Comparison of commission on cancer-approved and -nonapproved hospitals in the United States: implications for studies that use the National Cancer Data Base. J Clin Oncol 2009;27:4177-81. [Crossref] [PubMed]
- Boffa DJ, Rosen JE, Mallin K, et al. Using the National Cancer Database for Outcomes Research: A Review. JAMA Oncol 2017;3:1722-8. [Crossref] [PubMed]
- McAteer JP, Goldin AB, Healey PJ, et al. Hepatocellular carcinoma in children: epidemiology and the impact of regional lymphadenectomy on surgical outcomes. J Pediatr Surg 2013;48:2194-201. [Crossref] [PubMed]
- Lafaro KJ, Pawlik TM. Fibrolamellar hepatocellular carcinoma: current clinical perspectives. J Hepatocell Carcinoma 2015;2:151-7. [PubMed]
- Chaudhari VA, Khobragade K, Bhandare M, et al. Management of fibrolamellar hepatocellular carcinoma. Chin Clin Oncol 2018;7:51. [Crossref] [PubMed]
- Honeyman JN, Simon EP, Robine N, et al. Detection of a recurrent DNAJB1-PRKACA chimeric transcript in fibrolamellar hepatocellular carcinoma. Science 2014;343:1010-4. [Crossref] [PubMed]
- Cornella H, Alsinet C, Sayols S, et al. Unique genomic profile of fibrolamellar hepatocellular carcinoma. Gastroenterology 2015;148:806-18 e10.
- Darcy DG, Chiaroni-Clarke R, Murphy JM, et al. The genomic landscape of fibrolamellar hepatocellular carcinoma: whole genome sequencing of ten patients. Oncotarget 2015;6:755-70. [Crossref] [PubMed]
- Graham RP, Jin L, Knutson DL, et al. DNAJB1-PRKACA is specific for fibrolamellar carcinoma. Mod Pathol 2015;28:822-9. [Crossref] [PubMed]
- Xu L, Hazard FK, Zmoos AF, et al. Genomic analysis of fibrolamellar hepatocellular carcinoma. Hum Mol Genet 2015;24:50-63. [Crossref] [PubMed]


