
Association of family history with long-term prognosis in patients undergoing liver resection of HBV-related hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) is the most frequent histologic type of primary liver cancer, ranking 6th in incidence and 3rd in mortality worldwide (1). HCC is particularly prevalent in Africa and Southeast Asia, especially in China (2). More than 75% of cases worldwide and 85% of cases in developing countries have been attributed to hepatitis B virus (HBV) and hepatitis C virus (HCV), both of which increase the risk of HCC by approximately 20-fold (3). A familial aggregation of HCC has frequently been reported in Asians, particularly in China (4-9). A meta-analysis, based on 9 case-control and 4 cohort studies, demonstrated that the pooled relative risk for a family history of HCC was 2.50 [95% confidence interval (CI): 2.06–3.03] after adjusting for other confounding factors (10). This meta-analysis also reported that the combination of a family history of HCC and chronic HBV infection was associated with an over 70-fold elevated risk of HCC (10).
Despite convincing evidence that a family history of HCC is associated with HCC development, data on the association of family history with the long-term prognosis after HCC diagnosis and treatment are conflicting (11,12). One study reported that patients with HCC and a family history of HCC had better survival after multi-modalities treatment than patients without such a history (11). The authors postulated that the difference was due, in part, to earlier diagnosis, however certain genetic factors may also impact prognosis (11). Another study reported no association of family HCC history with long-term recurrence and survival after resection of HCC (12). These conflicting results may be due to different strategies in patient selection, as well as differences in the definitions of family history and treatment HCC modalities. In particular, unbalanced baseline characteristics (including demographic and clinicopathologic) among patients with and without a family history of HCC may have confounded comparisons.
The current study sought to examine the impact of a family HCC history relative to patient clinicopathologic characteristics, long-term recurrence and survival among patients undergoing curative-intent liver resection of HBV-related HCC. In particular, we sought to define the impact of family HCC history on long-term oncologic outcomes using propensity matched analysis of a large cohort of HCC patients.
Methods
Study population
Patients presenting to two departments of Hepatic Surgery, Eastern Hepatobiliary Surgery Hospital of Shanghai, the largest tertiary hepatobiliary center in China, between August 2003 and December 2013 were included. Inclusion criteria consisted of (I) 18 years of age or older, (II) medical history of chronic HBV infection, and a positive serology of Hepatitis B surface antigen (HBsAg), (III) newly diagnosed HCC without any previous treatment, and HCC was also confirmed by postoperative histopathological examination, (IV) curative-intent liver resection for HCC, which was defined as R0 resection, and (V) complete medical record on family history and other important prognostic variables. The study was approved by the Institutional Review Board of the Eastern Hepatobiliary Surgery Hospital of Shanghai, China.
Baseline characteristics and operative variables
Baseline patient characteristics and operative variables obtained from review of the medical records were included. Cirrhosis was confirmed by histopathological examination, and portal hypertension was defined as the presence of either esophageal varices, or splenomegaly with a decrease in platelet count (≤100×109/L). Tumor stage at diagnosis was determined following the Barcelona Clinic Liver Cancer (BCLC) staging system. Operative variables included intraoperative blood loss, requirement of blood transfusion, extent of hepatectomy, and type of liver resection. Major hepatectomy was defined as resection of three or more Couinaud liver segments; minor hepatectomy as resection of fewer than three segments. Anatomical resections were defined by the Brisbane 2000 Nomenclature of Liver Anatomy, while non-anatomical resections included wedge resection or limited resection.
Definition of family history
A family history of HCC was defined as a self-reported history of HCC in a first-degree relative. First-degree relatives included parents, siblings, or children, while nieces, nephews, aunts, uncles, or grandparents were excluded.
Use of antiviral therapy and follow-up
Among patients who had a preoperative HBV-DNA ≥1,000 copies/mL, adjuvant antiviral therapy with lamivudine 100 mg, adefovir dipivoxil 10 mg, or entecavir 0.5 mg orally daily was commenced immediately after surgery or after discharge. For patients with renal insufficiency, the daily lamivudine or adefovir dipivoxil dose was adjusted according to creatinine clearance.
The detailed follow-up schedule had previously been reported (13). In general, postoperative surveillance strategy for recurrence consisted of serum alpha-fetoprotein level, ultrasonography or contrast-enhanced computed tomography scan of the chest and abdomen at 2-monthly intervals for the first 6 months and at 3-monthly intervals thereafter. Computed tomography, magnetic resonance imaging, or positron emission tomography was performed when recurrence or distant metastasis was suspected. Tumor recurrence was defined as new appearance of intra- or extra-hepatic tumor nodules. Tumor recurrence was divided into early and late recurrences using a cut-off value of 2 years. The management of recurrence was based on the pattern of recurrent tumor, residual hepatic functional reserve, and general condition of the patient. The treatment included re-resection, local ablation therapy, liver transplantation, transcatheter arterial chemoembolization, oral sorafenib or supportive therapy. The dates of recurrence, last follow-up, and death were recorded.
Propensity score matching (PSM)
Patients with and without a family history of HCC were matched using PSM as described by Rubin and Rosenbaum (14,15). This was carried out using the R software version 3.1.0. The propensity score for an individual was calculated using a logistic regression model given the covariates included in Table 1. This method included ordering the case and control subjects, then selecting the first case subject and finding the control subject with the closest propensity score. Afterwards, both subjects were removed from consideration for matching and the next case subject was selected (16). The forward procedure was used, which started out with just the intercept and sequentially added the effect that most improved the fit. Variables were included up to a limit of a monotonized P-to-enter value of <0.2. Thereafter, a 1:1 nearest neighbor matching without replacement was performed so as to ensure any conditional bias was minimized.
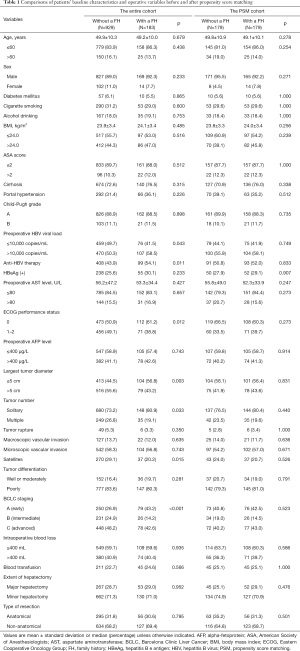
Full table
Statistical analysis
Baseline patient characteristics and operative variables among patients with and without a family history of HCC were summarized using frequency and percentage for categorical covariates, and mean ± standard deviation (SD) or median (range) for continuous covariates. Categorical and continuous covariates were compared using the Fisher’s exact test and the Wilcoxon rank-sum test, respectively. The primary outcome of the study was overall survival (OS), which was defined as the time from surgery to death resulting from any cause. The secondary outcome of this study was recurrence-free survival (RFS), which was defined as the time from surgery to tumor recurrence or occurrence of a new HCC, or death with evidence of recurrence. OS and RFS were compared among patients with and without a family history before and after PSM using the Kaplan-Meier curves and the log rank test. In order to adjust for other prognostic factors and enhance the accuracy of the model, a robust sandwich variance estimator in the multivariable Cox regression hazard regression analyses in the PSM cohort was used to estimate the hazard ratios and its 95% confidence interval. P<0.05 were considered statistically significant. Statistical analyses were carried out using the IBM SPSS Statistics version 25.0 and R software version 3.1.0.
Results
Among 1,541 patients who were screened, 429 patients did not fit the inclusion criteria and were excluded. The remaining 1,112 patients with chronic HBV infection who underwent curative liver resection for HCC were included in the final analytic cohort (Figure 1). There were 996 (89.6%) men and 116 (10.4%) women. The median age at operation was 50 years (range, 19–80 years). There were 183 (16.5%) patients who had a first-degree family history of HCC; 73.2% of patients had cirrhosis and 32.2% had portal hypertension.
Comparison of baseline characteristics and operative variables among patients with and without a family history of HCC are illustrated in Table 1. Several clinicopathological features were significantly different among patients with a family history versus patients without a history of HCC such as performance status, largest tumor diameter, tumor number, and presence of satellites (all P<0.05). Early HCC (BCLC A stage) among patients with a family history of HCC was also more common versus patients with no family history of HCC (43.2% vs. 26.9%, P<0.001).
PSM was used to create 179 pairs of patients. Patient characteristics and operative variables among patients with and without a family history after PSM are illustrated in Table 1. Of note, there were no differences in any of the baseline characteristics among patients with and without a family history of HCC (all P>0.2) after PSM.
Comparisons of long-term outcomes among patients with and without a family history of HCC are illustrated in Table 2. After PSM, there were no differences in early recurrence among patients with and without a family history (40.2% vs. 34.6%, P=0.326). However, the total recurrence, late recurrence, and mortality among patients with a family history were higher versus patients without such a history (75.4% vs. 53.6%, P<0.001, 35.2% vs. 19.0%, P=0.001, and 60.9% vs. 47.5%, P=0.015, respectively).
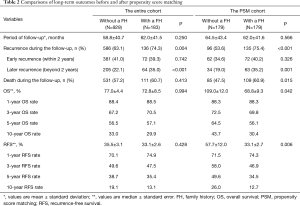
Full table
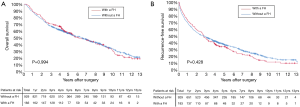
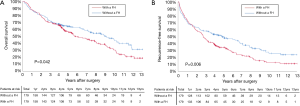
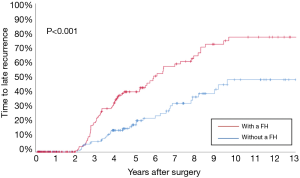
Before PSM, the 3-, 5-, and 10-year OS among patients with and without a family history of HCC were 70.5%, 57.1%, and 29.9%, and 67.2%, 56.5%, and 33.0%, respectively (Figure 2A). The 3-, 5-, and 10-year RFS among patients with and without a family history of HCC were 47.5%, 35.4%, and 13.1%, and 49.6%, 38.7%, and 19.1%, respectively (Figure 2B). Family history was not associated with increased risk of OS [hazard ratio (HR): 0.999; 95% CI: 0.814–1.226; P=0.994] and RFS (HR: 1.076; 95% CI: 0.897–1.292; P=0.428). After PSM, the 3-, 5-, and 10-year OS among patients with and without a family history of HCC were 69.8%, 56.1%, and 30.4%, and 72.5%, 64.5%, and 43.7%, respectively (Figure 3A). The 3-, 5-, and 10-year RFS among patients with and without a family history of HCC were 46.9%, 34.5%, and 12.7%, and 58.0%, 49.6%, and 26.0%, respectively (Figure 3B). Of note, after PSM, family history was associated with decreased risks of OS (HR: 1.342; 95% CI: 1.010–1.784; P=0.042) and RFS (HR: 1.420; 95% CI: 1.420–1.826; P=0.006). Figure 4 showed the comparisons of late recurrence (>2 years after surgery) rate between patients with and without a family history in the PSM cohort (P<0.001).
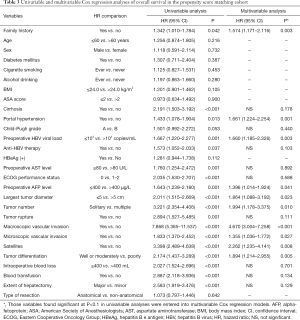
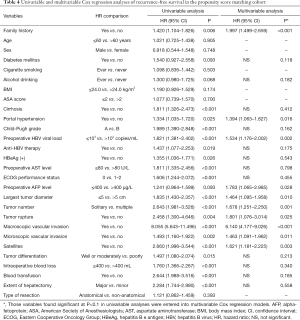
Univariable and multivariable Cox-regression analyses of OS and RFS after curative-intent liver resection of HBV-related HCC in the PSM cohort are shown in Tables 3,4. After univariable analysis, variables with P<0.1 were entered in the multivariable analysis. On multivariable Cox-regression analyses with robust estimator, after adjustment for other prognostic factors, a family history was independently associated with decreased OS and RFS after curative liver resection of HBV-related HCC. The adjusted HRs for OS and RFS were 1.574 (95% CI: 1.171–2.116; P=0.003) and 1.534 (95% CI: 1.176–2.002; P=0.002), respectively.
Full table
Full table
Discussion
In recent years, the association between family history and long-term oncologic prognosis has been studied in many malignant tumors, including colon cancer, stage III or IV gastric cancer, breast cancer, prostate cancer, and lung cancer (17-21). A large number of studies have identified that a family history of HCC increases the risk of HCC development and such a history increases the risk of developing HCC by 4- to 32-folds in patients with chronic HBV infection (4,7,10,22-27). However, very few studies have evaluated the impact of family history on long-term prognosis after liver resection of HCC. This large cohort study demonstrated that a family history of HCC was indeed associated with decreased OS and RFS after liver resection of HBV-related HCC, even after adjusting for the potential risk factors of patient’s demographic, environmental and clinicopathological characteristics.
Family history was based on self-reported information from the patients and only those patients with a family history of HCC in their first-degree relatives were enrolled and analyzed. The 2001 population-based Connecticut family health study noted that reports from first-degree relatives were more accurate than information from the second-degree relatives, with positive predictive values varying between 78% and 80% for lung and breast cancers (28). Numerous studies have also reported that only a family history of HCC in the first-degree relative increased the risk of developing HCC, but not the second-degree relatives (4,10,22). As a consequence, only patients with a family history of HCC in their first-degree relatives were enrolled in this study and second-degree relatives were excluded. In fact, 183 of 1,112 patients (16.4%) had a family history of HCC among their first-degree relatives. This proportion of patients with a family HCC history was similar to other reports. For example, a case-control study, Turati et al. (10) observed that 25 of 204 HCC patients (12.3%) had a first-degree relative with HCC in Western population of patients. In another study, Yu et al. (4) from Taiwan, observed that 17.5% of 553 male patients who had chronic HBV infection and HCC reported to a family history of HCC in a first-degree relative. Similarly, in another study from China, 169 of 1,313 HCC patients (12.9%) gave a history of HCC in a first-degree relative (12).
The strengths of the present study included the large sample size, the long-term follow-up, the standard definition used in the family history of HCC (restricted to the first-degree relatives), and the attempt to control for potential confounders by using the PSM method and multivariable Cox-regression analysis. PSM analysis was carried out to balance the differences in baseline variables among patients with and without a family history of HCC (16). After PSM, the real impact of family history on the prognosis of HCC after resection was more able to be determined. In addition, to further adjust for other confounding prognostic factors, a multivariable Cox regression analysis was applied to the PSM cohort. A family history was independently associated with decreased OS or RFS after resection for patients with HBV-related HCC. In the current study, rates of early recurrence (≤2 years after HCC resection) among patients with and without a family history appeared to be similar. Many previous studies have reported consistently that early recurrence within 2 years after resection is most likely the consequence of occult metastasis from the initial tumor, which is associated with aggressive tumor pathologic factors, such as large tumor size, multiple tumors, poor differentiation, macro- and microvascular invasion, and satellite lesions (29-31). In this way, it is understandable that family history was not associated with early recurrence after curative liver resection of HCC, as revealed by the result of this present study. However, the rate of late recurrence (>2 years after HCC resection) among patients with a family history was much higher (35.2% vs. 19.0%, P=0.001). Interestingly, the RFS curves among patients with or without a family history gradually separated after 2 years from the date of surgery (Figure 3B). These data suggest that late recurrence in patients with a family history may be higher than in patients without such a history. Apart from a small portion of late recurrence due to occult metastasis from the initial tumor, most late recurrence after 2 years of resection are commonly considered to develop from new malignant clones of HCC or de novo carcinogenesis (29). Thus, it is reasonable to assume that the genetic make-up of an individual contributes not only to the development of HCC, but also to the long-term prognosis after liver resection (28,32). Therefore, this significant difference in RFS, OS, and late recurrence between patients with and without a family history probably calls for closer and more stringent recurrence surveillance for patients with a family history in the late period of postresection follow-up, which may be helpful to early find and early treat those recurrent HCCs and improve the long-term prognosis after recurrence in our clinical practice.
A familial clustering of HCC in HBV carriers can be explained by shared HBV contagious infection among the first-degree relatives, inherent genetic contributions, and environmental or health-behavioral risk factors (4-9,25). In the present study, data on the environmental variables were prospectively collected such that their potential impact on tumor recurrence and survival after HCC resection could be investigated. Of note, there were no differences in the proportions of diabetes mellitus, cigarette smoking, alcohol drinking, and obesity among patients with and without a family history. Moreover, none of these factors were associated with RFS and OS after HCC resection, although some of these factors had been reported to be closely related to the development of HCC in previous epidemiological studies (7,32).
The analytic cohort in the current study consists of patients with HBV-related HCC. Previous studies by our group and others have demonstrated that preoperative HBV-DNA level over 10,000 copies/mL was an independent risk factor of OS and RFS after liver resection and anti-HBV therapy decreased HCC recurrence and prolonged survival for these patients (33,34). In the present study, a preoperative HBV-DNA level of over 10,000 copies/mL was indeed demonstrated to be an independent risk factor of OS and RFS. However, we did not find a beneficial impact of anti-HBV therapy on OS and RFS after PSM. The reasons for this difference are likely multifactorial and may be related to specific treatment regimens, treatment duration, discontinuation or adjustment after viral reactivation (35). It’s well-recognized that the eradication of HBV plays an important role in decreasing the development of HCC, as well as reducing HCC recurrence after curative resection. A recent randomized controlled trial study even showed that anti-HBV therapy can be effective for anti-recurrence in patients with low HBV-DNA load (<10,000 copies/mL) (35). However, in the study period [2003–2013], there were no guidelines in China even in the world which recommended antiviral therapy for those patients with low HBV load. The cost of antiviral drugs every day is not a small economic burden for most patients with chronic HBV infection in China as a developing country, especially for those who don’t have full health insurance. Although we recommend antiviral therapy for all patients with HBV-related HCC, regardless of preoperative HBV load, this issue is actually very complicated in the real clinical practice.
The current study had several limitations. Self-reporting of the family history was used and HCC family history was not confirmed with any relatives. This method may have resulted in under-reporting of the family history of HCC. As reporting of a family history is always more accurate for the first-degree relatives, we purposely did not extend to the second- and third-degree relatives. Although self-reporting the family history was limited to the first-degree relatives, this allowed greater accuracy (36,37). There was also a lack of information in the family history of chronic HBV infection in the first-degree relatives with HCC. HBV transmission among family members, together with other shared environmental risk factors, may be responsible for part of the observed familial aggregation of HCC. In addition, although the study indicated that the poor prognosis associated with a family history of HCC might be attributed to genetic contributions, defining the underlying mechanism was beyond the scope of the present study. Further studies involving tumor biology and genetic contributions in this group of patients with HCC and family history are required, especially for HBV-related HCC.
In conclusion, family history of HCC in a first-degree relative was associated with a worse OS and RFS after curative-intent resection among patients with HBV-related HCC. The genetic contributions of a family history might increase the risk of HCC recurrence after resection. To better assess HCC susceptibility in a population where HBV is endemic, future studies exploring the underlying mechanisms on the impact of genetic contributions on the development and recurrence of HCC in patients with chronic HBV infection are warranted.
Acknowledgements
Funding: This work was supported in part by the National Natural Science Foundation of China (No. 81472284 and 81672699 for T Yang).
Footnote
Conflicts of Interest: The abstract of this study has been presented as oral presentation at the 13th World Congress of the International Hepato-Pancreato-Biliary Association (4-7 Sep 2018, Gevena, Switzerland).
Ethical Statement: The study was approved by the Institutional Review Board of the Eastern Hepatobiliary Surgery Hospital of Shanghai, China.
References
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Donato F, Boffetta P, Puoti M. A meta-analysis of epidemiological studies on the combined effect of hepatitis B and C virus infections in causing hepatocellular carcinoma. Int J Cancer 1998;75:347-54. [Crossref] [PubMed]
- Yu MW, Chang HC, Liaw YF, et al. Familial risk of hepatocellular carcinoma among chronic hepatitis B carriers and their relatives. J Natl Cancer Inst 2000;92:1159-64. [Crossref] [PubMed]
- Chen CH, Huang GT, Lee HS, et al. Clinical impact of screening first-degree relatives of patients with hepatocellular carcinoma. J Clin Gastroenterol 1998;27:236-9. [Crossref] [PubMed]
- Sun Z, Lu P, Gail MH, et al. Increased risk of hepatocellular carcinoma in male hepatitis B surface antigen carriers with chronic hepatitis who have detectable urinary aflatoxin metabolite M1. Hepatology 1999;30:379-83. [Crossref] [PubMed]
- Chen CJ, Liang KY, Chang AS, et al. Effects of hepatitis B virus, alcohol drinking, cigarette smoking and familial tendency on hepatocellular carcinoma. Hepatology 1991;13:398-406. [Crossref] [PubMed]
- Yang Y, Wu QJ, Xie L, et al. Prospective cohort studies of association between family history of liver cancer and risk of liver cancer. Int J Cancer 2014;135:1605-14. [Crossref] [PubMed]
- Shen FM, Lee MK, Gong HM, et al. Complex segregation analysis of primary hepatocellular carcinoma in Chinese families: interaction of inherited susceptibility and hepatitis B viral infection. Am J Hum Genet 1991;49:88-93. [PubMed]
- Turati F, Edefonti V, Talamini R, et al. Family history of liver cancer and hepatocellular carcinoma. Hepatology 2012;55:1416-25. [Crossref] [PubMed]
- Dai WC, Fan ST, Cheung TT, et al. The impact of family history of hepatocellular carcinoma on its patients' survival. Hepatobiliary Pancreat Dis Int 2012;11:160-4. [Crossref] [PubMed]
- Huang J, Zhang Y, Chen M, et al. Family history of hepatocellulcar carcinoma is not associated with its patients' prognosis after hepatectomy. World J Surg Oncol 2013;11:280. [Crossref] [PubMed]
- Yang T, Lu JH, Lau WY, et al. Perioperative blood transfusion does not influence recurrence-free and overall survivals after curative resection for hepatocellular carcinoma: A Propensity Score Matching Analysis. J Hepatol 2016;64:583-93. [Crossref] [PubMed]
- Rubin DB, Thomas N. Matching using estimated propensity scores: relating theory to practice. Biometrics 1996;52:249-64. [Crossref] [PubMed]
- Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat 1985;39:33-8.
- D'Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998;17:2265-81. [Crossref] [PubMed]
- Chan JA, Meyerhardt JA, Niedzwiecki D, et al. Association of family history with cancer recurrence and survival among patients with stage III colon cancer. JAMA 2008;299:2515-23. [Crossref] [PubMed]
- Han MA, Oh MG, Choi IJ, et al. Association of family history with cancer recurrence and survival in patients with gastric cancer. J Clin Oncol 2012;30:701-8. [Crossref] [PubMed]
- Thalib L, Wedrén S, Granath F, et al. Breast cancer prognosis in relation to family history of breast and ovarian cancer. Br J Cancer 2004;90:1378-81. [Crossref] [PubMed]
- Ganti AK, Loberiza FR, Kessinger A. Association of positive family history with survival of patients with lung cancer. Lung Cancer 2009;63:136-9. [Crossref] [PubMed]
- Westerman ME, Gershman B, Karnes RJ, et al. Impact of a family history of prostate cancer on clinicopathologic outcomes and survival following radical prostatectomy. World J Urol 2016;34:1115-22. [Crossref] [PubMed]
- Hassan MM, Spitz MR, Thomas MB, et al. The association of family history of liver cancer with hepatocellular carcinoma: a case-control study in the United States. J Hepatol 2009;50:334-41. [Crossref] [PubMed]
- Donato F, Gelatti U, Chiesa R, et al. A case-control study on family history of liver cancer as a risk factor for hepatocellular carcinoma in North Italy. Brescia HCC Study. Cancer Causes Control 1999;10:417-21. [Crossref] [PubMed]
- Lin YY, Yu MW, Lin SM, et al. Genome-wide association analysis identifies a GLUL haplotype for familial hepatitis B virus-related hepatocellular carcinoma. Cancer 2017;123:3966-76. [Crossref] [PubMed]
- Loomba R, Liu J, Yang HI, et al. Synergistic effects of family history of hepatocellular carcinoma and hepatitis B virus infection on risk for incident hepatocellular carcinoma. Clin Gastroenterol Hepatol 2013;11:1636-45.e1-3.
- Zhang YQ, Peng LJ, Cao YR, et al. Risk Factors for Hepatocellular Carcinoma in Cirrhotic Patients with Chronic Hepatitis B. Genet Test Mol Biomarkers 2016;20:535-43. [Crossref] [PubMed]
- Tong MJ, Huynh TT, Siripongsakun S. Familial clustering of hepatocellular carcinoma in HBsAg-positive patients in the United States. Hepatol Int 2013;7:1019-29. [Crossref] [PubMed]
- Yuan JM, Lu SC, Van Den Berg D, et al. Genetic polymorphisms in the methylenetetrahydrofolate reductase and thymidylate synthase genes and risk of hepatocellular carcinoma. Hepatology 2007;46:749-58. [Crossref] [PubMed]
- Xu XF, Xing H, Han J, et al. Risk Factors, Patterns, and Outcomes of Late Recurrence After Liver Resection for Hepatocellular Carcinoma: A Multicenter Study From China. JAMA Surg 2018. [Epub ahead of print]. [PubMed]
- Imamura H, Matsuyama Y, Tanaka E, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol 2003;38:200-7. [Crossref] [PubMed]
- Portolani N, Coniglio A, Ghidoni S, et al. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg 2006;243:229-35. [Crossref] [PubMed]
- Volk ML, Lok AS. Is family history of liver cancer a risk factor for hepatocellular carcinoma. J Hepatol 2009;50:247-8. [Crossref] [PubMed]
- Yang T, Lu JH, Zhai J, et al. High viral load is associated with poor overall and recurrence-free survival of hepatitis B virus-related hepatocellular carcinoma after curative resection: a prospective cohort study. Eur J Surg Oncol 2012;38:683-91. [Crossref] [PubMed]
- Huang G, Lau WY, Wang ZG, et al. Antiviral therapy improves postoperative survival in patients with hepatocellular carcinoma: a randomized controlled trial. Ann Surg 2015;261:56-66. [Crossref] [PubMed]
- Huang G, Li PP, Lau WY, et al. Antiviral Therapy Reduces Hepatocellular Carcinoma Recurrence in Patients With Low HBV-DNA Levels: A Randomized Controlled Trial. Ann Surg 2018;268:943-54. [Crossref] [PubMed]
- Mai PL, Garceau AO, Graubard BI, et al. Confirmation of family cancer history reported in a population-based survey. J Natl Cancer Inst 2011;103:788-797. [Crossref] [PubMed]
- Kerber RA, Slattery ML. Comparison of self-reported and database-linked family history of cancer data in a case-control study. Am J Epidemiol 1997;146:244-8. [Crossref] [PubMed]


