
Significance of preoperative sarcopenia to liver surgery
Sarcopenia, characterized by a decline in skeletal muscle mass and muscle strength or physical activity, is now accepted worldwide as a new geriatric syndrome. Recent studies have identified significant associations between sarcopenia and poor outcomes of various diseases including liver diseases (1-3). This article reviews the significance of preoperative sarcopenia to liver surgery, particularly liver transplantation (LT).
Aging causes primary sarcopenia, and other conditions such as disuse, malnutrition, organ failure, cancer and surgery cause secondary sarcopenia. In fact, most patients who undergo liver surgery present with a number of these factors related to sarcopenia. Due to the aging of societies, more patients will be undergoing hepatectomy for hepatocellular carcinoma (HCC) and metastatic liver tumor later in life. Far more patients are presently octogenarians, even compared with only a few decades ago. Most patients who undergo liver resection have cancer and malnutrition and most of those waitlisted for LT have liver failure, malnutrition and disuse due to massive ascites and hepatic encephalopathy. Furthermore, all such patients eventually must undergo surgery, which itself causes sarcopenia. Therefore, patients undergoing liver surgery are highly likely to have sarcopenia. Undoubtedly, sarcopenia is an important issue to consider in liver surgery.
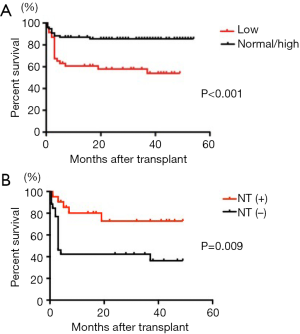
We previously showed worse rates of surviving living donor LT (LDLT), among patients with preoperative low skeletal muscle mass assessed using a body composition analyzer compared with those whose skeletal muscle mass was normal or high (Figure 1A) (4). However, perioperative nutritional therapy significantly increased overall survival (OS) in patients with low skeletal muscle mass (Figure 1B). We also found that preoperative poor muscle quality evaluated as intramuscular adipose tissue content (IMAC) was an independent risk factor for death after LDLT as well as low skeletal muscle mass assessed by preoperative computed tomography (CT) (5). Based on these findings, we started to revise our indications for LDLT (except under circumstances of acute liver failure) in 2013 with the aim of excluding patients with severe sarcopenia who are able to walk unaided (6). Historically, patients unable to walk unaided due to severe liver failure and malnutrition have been referred to our tertiary LT center from other LT institutions.
Sarcopenia is defined as a decrease in skeletal muscle mass and muscle strength or physical activity (7,8). However, most studies to date have only considered low skeletal muscle mass as sarcopenia. Therefore, we prospectively evaluated sarcopenia in patients undergoing LDLT by measuring muscle handgrip strength in addition to skeletal muscle mass, and examined the validity of these criteria (6). Among 72 adult patients, 10 (14%) were defined as having pretransplant sarcopenia. OS rates were significantly lower in patients with, than without sarcopenia (P<0.001). This study prospectively showed that preoperative sarcopenia defined as muscle strength and skeletal mass according to our criteria significantly and negatively impacted survival after LDLT. A one-year OS rate of 94% for all of our patients validated our new criteria. Moreover, skeletal muscle mass worsened after LDLT and did not return to preoperative levels until one year after LDLT. By contrast, handgrip strength sharply decreased at one month after LDLT, but returned to preoperative levels by six months thereafter. This finding suggests that not only short-term, but also mid- to long-term nutritional and rehabilitation intervention is necessary to restore body composition after LT.
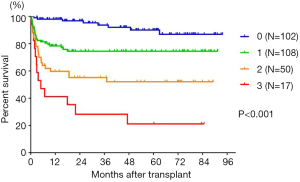
Skeletal muscle mass can be evaluated by cross-sectional imaging modalities such as CT at the L3 level or assessing body composition using bioimpedance analysis (BIA) and dual X-ray absorptiometry (DXA). Among these modalities, CT is the most useful for patients undergoing liver surgery, because such patients preoperatively took abdominal CT to evaluate liver tumor and portal vein thrombosis, which avoids additional exposure to radiation and the higher costs of CT to assess skeletal muscle mass. However, sarcopenia working groups in Europe and Asia have not yet proposed cut-off values for skeletal muscle mass determined by CT, whereas some have been proposed for BIA and DXA assessments (7,8). Data from healthy individuals are needed to establish sex-specific cut-off values for low skeletal muscle mass appropriate to a definition of sarcopenia. However, healthy persons have no need of CT. Therefore, we studied healthy living donors who are routinely assessed by preoperative abdominal CT. We recently proposed a sex-specific cut-off for a skeletal muscle mass index (SMI), IMAC, and a visceral-to-subcutaneous adipose tissue area ratio (VSR) based on data derived from 657 healthy donors for LDLT (9). Abnormal factors such as low SMI (low muscle mass), high IMAC (low muscle quality), and high VSR (visceral adiposity) additively contributed to an increased risk of post-LDLT mortality (Figure 2) (9). The lowest one-year survival rate after LDLT was 41.2% (P<0.001) in 17 (6.1%) patients who were beyond all three cut-offs. Based on this finding, we established and implemented objective criteria for recipients of LDLT or split liver LT in deceased donor LT (DDLT) in October 2016 to exclude patients with abnormalities in all three factors, because the previous criterion of being able to walk unaided is subjective. Alternatively, we recommend that patients with abnormalities in all three factors register for DDLT, because body composition did not significantly impact outcomes of DDLT (data not shown). We provide aggressive perioperative nutrition and rehabilitation therapy to improve the outcomes of LDLT recipients with one or two abnormal factors. Consequently, the one-year survival after LDLT was 98% (October 2018). Our strategy based on preoperative body composition resulted in excellent short-term outcomes after LDLT.
We recently reported that preoperative high VSR, low SMI and high IMAC together with known risk factors such as high alpha-fetoprotein and advanced tumor stage, are independent risk factors for mortality and HCC recurrence among patients undergoing hepatectomy for HCC (10). Moreover, preoperative muscle steatosis evaluated using IMAC closely correlated with an increased incidence of postoperative (particularly infectious) complications (11). We also clarified that low SMI, poor muscle quality and visceral adiposity are closely related to mortality after intrahepatic cholangiocarcinoma (ICC) resection (12). We also recently showed that preoperative sarcopenic obesity (having both obesity and sarcopenia), is an independent risk factor for death and HCC recurrence after hepatectomy for HCC (13). Therefore, the negative impacts of preoperative sarcopenia and visceral adiposity on outcomes are likely to be universal for all types of liver surgery.
The mechanisms through which low muscle skeletal mass, muscle quality, and visceral adiposity adversely affect postoperative morbidity, mortality, and HCC recurrence remains unclear. Skeletal muscle and adipose tissue are regarded as secretory organs. Myocytes produce and release various physiologically crucial cytokines and proteins, called myokines, and adipocytes produce several proinflammatory cytokines and adipokines. Accumulated visceral fat and intramuscular adipose tissue increase levels of proinflammatory cytokines and adipokines that are associated with chronic inflammation and carcinogenesis. In addition, adipose tissue is a rich source of adipose-derived stem cells that can promote cancer, influence the tumor microenvironment and promote angiogenesis. Muscle wasting results in decreased production of interleukin (IL)-15, which plays a crucial role in reversing insulin resistance and enhancing the development, survival, and activation of natural killer (NK) cells (14). Furthermore, increased amounts of adipokines, such as leptin, IL-6, and tumor necrosis factor shorten NK cells survival or stimulate NK cells to produce pro-inflammatory IL-17 (15). These interrelations between myokines and adipokines might be associated with impaired survival and recurrent HCC and ICC in patients with sarcopenia. We are presently investigating the effect of preoperative sarcopenia and visceral adiposity on cancer recurrence and immunological status in experimental models.
In conclusion, preoperative sarcopenia and abnormal body composition exert a powerful negative effect on outcomes after liver surgery. Therefore, preoperative assessment of body composition in addition to the standard preoperative workup is crucial. Moreover, appropriate pre- and postoperative nutritional support and rehabilitation programs should be established for patients undergoing liver surgery to improve outcomes, even when preoperative body composition is impaired.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Meza-Junco J, Montano-Loza AJ, Baracos VE, et al. Sarcopenia as a prognostic index of nutritional status in concurrent cirrhosis and hepatocellular carcinoma. J Clin Gastroenterol 2013;47:861-70. [Crossref] [PubMed]
- Voron T, Tselikas L, Pietrasz D, et al. Sarcopenia Impacts on Short- and Long-term Results of Hepatectomy for Hepatocellular Carcinoma. Ann Surg 2015;261:1173-83. [Crossref] [PubMed]
- Reisinger KW, van Vugt JL, Tegels JJ, et al. Functional compromise reflected by sarcopenia, frailty, and nutritional depletion predicts adverse postoperative outcome after colorectal cancer surgery. Ann Surg 2015;261:345-52. [Crossref] [PubMed]
- Kaido T, Ogawa K, Fujimoto Y, et al. Impact of sarcopenia on survival in patients undergoing living donor liver transplantation. Am J Transplant 2013;13:1549-56. [Crossref] [PubMed]
- Hamaguchi Y, Kaido T, Okumura S, et al. Impact of quality as well as quantity of skeletal muscle on outcomes after liver transplantation. Liver Transpl 2014;20:1413-9. [Crossref] [PubMed]
- Kaido T, Tamai Y, Hamaguchi Y, et al. Effects of pretransplant sarcopenia and sequential changes in sarcopenic parameters after living donor liver transplantation. Nutrition 2017;33:195-8. [Crossref] [PubMed]
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412-23. [Crossref] [PubMed]
- Chen LK, Liu LK, Woo J, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2014;15:95-101. [Crossref] [PubMed]
- Hamaguchi Y, Kaido T, Okumura S, et al. Proposal for new selection criteria considering pre-transplant muscularity and visceral adiposity in living donor liver transplantation. J Cachexia Sarcopenia Muscle 2018;9:246-54. [Crossref] [PubMed]
- Hamaguchi Y, Kaido T, Okumura S, et al. Preoperative visceral adiposity and muscularity predict poor outcomes after hepatectomy for hepatocellular carcinoma. Liver Cancer 2018. [Epub ahead of print]. [Crossref]
- Hamaguchi Y, Kaido T, Okumura S, et al. Muscle Steatosis is an Independent Predictor of Postoperative Complications in Patients with Hepatocellular Carcinoma. World J Surg 2016;40:1959-68. [Crossref] [PubMed]
- Okumura S, Kaido T, Hamaguchi Y, et al. Impact of Skeletal Muscle Mass, Muscle Quality, and Visceral Adiposity on Outcomes Following Resection of Intrahepatic Cholangiocarcinoma. Ann Surg Oncol 2017;24:1037-45. [Crossref] [PubMed]
- Kobayashi A, Kaido T, Hamaguchi Y, et al. Impact of Sarcopenic Obesity on Outcomes in Patients Undergoing Hepatectomy for Hepatocellular Carcinoma. Ann Surg 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Mishra A, Sullivan L, Caligiuri MA. Molecular pathways: interleukin-15 signaling in health and in cancer. Clin Cancer Res 2014;20:2044-50. [Crossref] [PubMed]
- Lutz CT, Quinn LS. Sarcopenia, obesity, and natural killer cell immune senescence in aging: altered cytokine levels as a common mechanism. Aging (Albany NY) 2012;4:535-46. [Crossref] [PubMed]


