Multidisciplinary management of recurrent and metastatic hepatocellular carcinoma after resection: an international expert consensus
Introduction
Hepatocellular carcinoma (HCC) is the sixth-most common cancer and the third leading cause of cancer-related death in the world, with more than 50% of new cases being diagnosed in China (1). According to the latest data from China (2), HCC is the fourth-most common malignancy and the third leading cause of mortality. Surgical treatment, including hepatectomy and liver transplantation, is the most commonly used approach to improve the survival of patients. However, 40–70% patients eventually suffer from postoperative recurrence within 5 years (3). Nevertheless, there is an opportunity to improve patient prognosis if investigators can recognize the importance of a standardized perioperative management and study it in clinical and pre-clinical settings. Preoperative evaluation, recurrence prediction, surgical technique, postoperative surveillance and treatment should be standardized for HCC management. A multidisciplinary team (MDT) could thus maximize the advantages of different disciplines and benefits to patients. Based on our own experience and published studies from other researchers, for the first time, we have reached a consensus for the management of recurrence and metastasis after HCC resection. A draft consensus was written by the MDT of West China Hospital. During the preparation of the consensus, all important aspects of MDT management of HCC were discussed with other professors specializing in liver surgery, hepatic tumor, hepatitis and hepatic imaging from West China Hospital. After that, more experts from renowned hospitals in other regions of China joined to update the consensus. We also invited experts from Italy, Korea, Japan and the USA to review and improve the consensus, thus formulating an international consensus. With emerging evidence, this initial version of the consensus needs to be updated and improved in the future.
According to the accepted practice, the grades of evidences are presented in Table 1 (4). The strength of recommendations is showed in Table 2 (5).

Full table

Full table
Consensus recommendations
Diagnostic criteria and preoperative evaluation of HCC and recurrent HCC (RHCC)
Clinical diagnostic criteria for HCC
It has been recognized that HCC is the only solid tumor for which clinical diagnostic criteria are adopted. In clinically diagnosing HCC, three factors are considered: underlying chronic liver disease, imaging features and serum alpha-fetoprotein (AFP) level. Presently, the internationally recognized clinical diagnosis standards, i.e., meeting 1.1.1+1.1.2.1+1.1.3 or 1.1.1+1.1.2.2 or a biopsy of a suspicious lesion, should be implemented (3,6-11).
History of hepatitis and/or cirrhosis
Evidence of cirrhosis and hepatitis B virus (HBV) and/or hepatitis C virus (HCV) infection (positive for HBV and (or) HCV antigen). History of nonalcoholic fatty liver disease (NAFLD)
Typical HCC imaging features
Magnetic resonance imaging (MRI) and/or computed tomography (CT)-enhanced arterial scan and/or enhanced multi-phase scan indicating that an intrahepatic lesion is inhomogeneous or homogeneous enhancement during arterial phase, with venous or delayed phase washout (12-16). The definitions of techniques, structure and categorization embodied by LI RADS—developed by the American College of Radiology is helpful in defining the imaging findings associated with HCC and RHCC (17).
If the diameter of the hepatic lesion is 1–2 cm, HCC can be diagnosed only when CT and MRI examinations both indicate typical imaging features of HCC. If the diameter of the liver lesion is more than 2 cm, HCC can be diagnosed when either CT or MRI examination indicates the typical imaging features corresponding to HCC.
Increased level of AFP
Serum AFP ≥400 µg/L for 1 month or ≥200 µg/L for 2 months, and when the increase in AFP level due to other reasons can be excluded, including pregnancy, germline embryonic tumor, active liver disease and secondary liver carcinoma. The use of GALAD and BALAD scores have demonstrated significantly improved detection of early stage HCC and are becoming increasingly popular to help determine the likelihood of HCC (18,19).
Criteria of pathologic diagnosis
Pathologic examination is the gold standard for diagnosis. Samples obtained from the biopsy of a liver-occupying lesion or extrahepatic metastasis or specimens excised by surgery can be diagnosed as HCC by cytologic and (or) histopathologic examination.
Diagnostic criteria of RHCC
Similar to the diagnostic criteria for primary HCC, imaging examinations displaying typical HCC vasculature are required when diagnosing RHCC (3,7,20-24). Two or even three kinds of imaging examinations can complement one another (3), which is of great significance in accurate staging, prognostic prediction and optimal treatment selection for HCC management. The application of previous therapeutic modalities such as transarterial chemoembolization (TACE) and transarterial radioembolization (TARE) can make it very difficult to determine the presence of RHCC using a single modality. Imaging over several months also dramatically assists in the radiographic diagnosis of RHCC.
In the presence of an underlying chronic liver disease, Gd-EOB-DTPA-enhanced MRI is recommended to identify posterior necrotic foci, hemorrhagic foci, regenerative nodules and HCC (12-16,25).
Preoperative tumor marker levels, imaging characteristics and other risk factors to predict recurrence after surgery
Preoperative AFP level: The prognosis of patients with apparent AFP increase (≥400 µg/L) is poorer than the prognosis of patients with no AFP increase or slight AFP increase (26-28).
Imaging characteristics: The prognosis of patients with HCC accompanied by growth outside the capsule, multiple nodules with fusion growth or with HCC without capsule is poorer than the prognosis of patients with single-nodule HCC (29,30). Imaging studies indicated that a single HCC lesion with a diameter greater than 5 cm or multi-nodule HCC is associated with a high incidence of microvascular invasion (MVI) (31,32). Additionally, the presence of arterial vessels in tumors on imaging examinations is a risk factor for MVI (33). Likewise, the presence of portal vein tumor thrombus (PVTT) in imaging examinations is indicative of poor prognosis (34,35). Patients with PVTT and (or) lymph node metastasis usually do not benefit from liver transplantation (36). Furthermore, patients whose preoperative imaging examinations indicate 4 or more HCC nodules fail to acquire survival benefit from resection (37).
High expression of HCC stem cell markers (38) and EpCAM-CTC7.5 ≥2 (39) indicate poor prognosis.
HCC patients with increased levels of AFP, AFP isoforms (AFP-L3) and des-gamma-carboxy prothrombin (DCP) before surgery have poor prognosis (40). Therefore, combining tumor size, serum DCP levels and standardized uptake value (SUVmax) in positron emission tomography (PET)/CT examinations can precisely predict MVI. For example, when the tumor diameter was 3.6 cm, DCP level was 101 mAU/mL and SUVmax in PET/CT examination was above 4.2, the sensitivity and specificity of MVI prediction were 100% and 90.9%, respectively (41).
Measures of recurrence prevention during hepatectomy
For large lesions in the right or left liver lobe, especially in the presence of invasion of diaphragm assessed by preoperative imaging, hepatectomy via the anterior approach should be implemented (42).
If blood loss is anticipated to be 600–800 mL during surgery, hepatic inflow occlusion or half-hepatic inflow occlusion should be performed (43,44). There is no evidence demonstrating that hepatic inflow occlusion adversely affects long-term prognosis (45); however, excessive blood loss is a risk factor for reduced survival (46).
Intraoperative ultrasound or ultrasound contrast imaging (47) during operation can further identify satellite nodules, tumor emboli and lesions in the remnant liver. The relation between lesion and first, second and third hepatic portis can be re-evaluated. In addition, intraoperative ultrasound or ultrasound contrast imaging can help determine the resection line and margin.
Confirmed or suspicious lesions found during operation should be simultaneously resected or ablated (48).
Anatomical hepatectomy should be the optimal choice based on the anticipated remnant liver volume, ICG R15 index, cirrhosis degree and tumor extension. Based on these factors, non-anatomical hepatectomy or local resection with a wide resection margin could be considered (49,50). In patients with liver cirrhosis or simple nodular type HCC with close proximity to the major vasculature, marginal resection can be considered securing sufficient future liver remnant volume (51).
Radical resection criteria for HCC (3,22,47,52)
Intraoperative evaluation
- No invasion to adjacent organs or lymphatic and distant metastasis;
- All the tumors can be completely resected. Resection margin >1 cm is preferable; however, if the margin is <1 cm, no residual tumor cells are found at resected cross section;
- The tumor number does not exceed three by intraoperative ultrasound scanning. For patients with 4 or more tumors, TACE or radiotherapy should be considered, rather than proceed to resection.
Postoperative evaluation from pathological reports
Standard pathological sampling and reports (32) should be adopted. The presence or absence of MVI and satellite nodules, and surgical margin should be mentioned on the pathological report.
Postoperative evaluation from surveillance 2 months after operation (3)
- Ultrasound scan, CT scan or MRI (two scans are necessary) should be performed 2 months after operation;
- Quantitative determination of AFP should be performed 2 months after operation. The duration for the AFP level to become negative is more than 2 months for a small proportion of patients.
Management of patients who have a risk of recurrence after operation
Identification of patients at risk of recurrence
Risk factors for recurrence should be re-evaluated 1 to 2 months after operation according to dynamic changes in blood cell counts, AFP and DCP levels, and surgical outcomes and pathological reports. For example, poor survival is usually observed if the following three risk factors are simultaneously present: platelet to lymphocyte ratio (PLR) <107, presence of MVI and tumor diameter ≥6.8 cm (53). There are several risk factors of recurrence after operation including the following:
- Surgical factors: non-anatomical resection (only for 2–5cm HCC) (54), positive histologic margin (55), substantial blood loss, need for transfusion (56) and iatrogenic tumor escape/rupture;
- Clinicopathological factors: poorly differentiated tumor, advanced tumor stage, tumor rupture (57), no intact capsule, tumor diameter >5 cm, tumor number ≥3 (58), vessel invasion (including vascular and bile duct tumor thrombus) (59), lymph node metastasis (60), satellite lesion, adjacent organ invasion, high level of AFP before operation (59), increased AFP level 2 months after operation (61);
- Underlying liver disease: active hepatitis infection and cirrhosis.
Recommendation 1
Presence of macroscopic tumor thrombus, MVI, multiple tumors, satellite nodule or lymph node metastasis and lack of change of AFP level from positive to negative at 2 months after operation are clear indicators of a high risk of recurrence; in these cases, postoperative adjuvant therapy might be considered (evidence level II b; recommendation C)
Postoperative surveillance
HCC patients should be regularly monitored after operation. Liver imaging examination, expression of tumor markers (AFP and DCP), HBV-DNA level, blood cell count and liver function should be evaluated (6,7,62).
Recommendation 2
Follow-up should be performed every 3 to 4 months within the first 2 years after operation. If all evaluated factors remain normal for 5 years, the follow-up interval could be increased to 6 months (evidence level IV; recommendation B).
Treatment for patients according to the risk of tumor recurrence
Present evidences (63-65) show that inappropriate adjuvant therapy, such as TACE, for patients without a high risk of recurrence, could possibly damage the remnant liver, which could lead to liver function deterioration, adversely affect long-term survival and even increase the incidence of extrahepatic metastasis.
Recommendation 3
Except for systematic antiviral therapy for HBV- and/or HCV-related HCC, postoperative adjuvant therapy is not recommended for patients without recurrent risks (evidence level IV; recommendation B).
For patients who are at risk of recurrence after operation, no widely recognized treatment is recommended. Several studies demonstrate that for patients with vessel invasion, multiple lesions and tumor diameter >5 cm, postoperative TACE could be beneficial for survival.
TACE (34,63,66-69), antiviral therapy (70-72), immunomodulation therapy, such as thymosin α1 (73-75) or interferon (76-79), sorafenib (80-82) and vitamin K2 (83-87), could be considered for patients who are at risk of recurrence. Alternatively, combined chemotherapy could be considered for these patients (88,89).
Recommendation 4
The following postoperative therapies may benefit for patients who are at risk of recurrence which are systematic antiviral therapy for HBV-related HCC (evidence level Ia; recommendation A), interferon (evidence level Ia; recommendation B), TACE (evidence level Ib; recommendation B), sorafenib (evidence level Ib; recommendation C), vitamin K2 (evidence level Ib; recommendation C) and thymosin α1 (evidence level IIb; recommendation C).
Intrahepatic recurrence pattern and clinical significance after resection
It is generally recognized that intrahepatic RHCC may have a monoclonal (or monocentric) origin when it develops from an intrahepatic metastasis (IM) or have a multiclonal (or multicentric) origin (MO) when it arises from de novo carcinogenesis due to long-term chronic inflammation and cirrhosis from HBV or HCV infection.
The earliest identification of RHCC is based on clinicopathological characteristics. Recurrence occurring within 1 year of surgery is typically defined as IM, while recurrence occurring later than 1 year after resection is defined as MO RHCC (90). IM can also be identified based on pathologic diagnosis (91), whereas MO RHCC can be identified based on tumor differentiation (92,93). However, the sensitivity and specificity of these techniques are not optimal, because the results can be greatly influenced by subjective factors pertaining to the examining pathologist(s). With the development of molecular biotechnologies and genomic technologies, clinicians and pathologists have explored multiple diagnostic approaches for identifying the origin of RHCC, including loss of heterozygosity (LOH) analysis, microsatellite instability detection, TP53 gene mutation analysis, X chromosome inactivation analysis, HBV-DNA integration detection, DNA methylation analysis, microRNA (miRNA) spectrum analysis and comparative genomic hybridization (CGH) (94-103). By using multi-omics methods and combining clinicopathological characteristics, some scholars (104,105) have explored and identified tumor heterogeneity and origins of multiple-nodule HCC. Among these methods, the detection of LOH has been widely employed to identify the origins of RHCC. Microsatellite DNA is a good marker of the integral stability of DNA. The detection of multiple chromosomes that have a high-frequency of LOH may improve the accuracy of the identification of RHCC origin. Additionally, the required specimen can be easily obtained, because formaldehyde-fixed and paraffin-embedded samples or biopsy samples satisfy the detection requirements of this technique (94).
Recommendation 5
RHCC with different origins should be distinctly treated. The overall survival (OS) of patients with MO RHCC may be better than that of patients with IM. For patients with IM, intervention therapy or targeted drugs may be beneficial, while second resection or liver transplantation may provide comparable curative effect as the initial resection for patients with MO RHCC (evidence level IIb; recommendation B).
Surgical treatment for patients with intrahepatic recurrence after resection
Repeat resection for resectable RHCC
Studies (106,107) have demonstrated that for patients with resectable RHCC after resection, the OS is better after repeat resection than that after TACE. Similar conclusions have also been obtained from a systematic review (108). Some studies (109,110) have demonstrated that patients can even benefit from a third hepatectomy but that more than three repeated hepatectomy cannot improve survival (111). Additionally, surgical resection is beneficial for resectable extrahepatic metastasis (112). The prognosis of RHCC patients after repeat resection was found to be closely associated with the clinicopathologic characteristics of primary HCC and recurrence interval (113). Repeat resection is usually better for patients with a single tumor without vascular invasion and with a recurrence interval ≥1 year (113,114).
Preoperative evaluation for patients with resectable RHCC
Preoperative evaluation for RHCC is usually similar to that before initial operation, in which exclusion of distant metastasis, the performance status (PS), liver function, hepatic reserve function, cirrhosis degree, portal hypertension and future liver volume (FLV) (109,115-117) are considered. For repeated resection, the PS score of patients should be 0–1, Child-Pugh staging should be class A or recovering to class A from B after short-term therapy before surgery, the liver reserve function should be normal, and there should be no apparent dysfunction of other organs. The FLV of patients should be comprehensively considered according to their liver function, liver reserve function and other indicators (118). For patients with cirrhosis, the FLV should be greater than 40% if ICG R15 <10%, while the FLV should be greater than 50% if ICG R15 is 10–20% (119-121). Additionally, for patients with liver fibrosis, the FLV should be greater than 30%, and for patients without an underlying liver disease, the FLV should be greater than 20% (122). Repeated resection may be safe and feasible for older patients if their clinical conditions have been strictly evaluated (123).
Compared with traditional laparotomy, laparoscopic hepatectomy is characterized as being minimally invasive and to have shorter recovery time (124). Many studies (125-129) have indicated that there are no significant differences in disease-free survival (DFS) and OS between RHCC patients undergoing laparoscopic operation and traditional laparotomy. However, it should be noted that in some of these studies, there may have been a selection bias regarding tumor size and location before laparoscopic operation (126,127,130).
Recommendation 6
RHCC patients could benefit from a second or a third resection. However, before surgery, the liver function, liver reserve function and FLV should be strictly evaluated (evidence level IIb; recommendation B). Laparoscopic hepatectomy could be performed at experienced centers. To avoid unnecessary conversion, the relevant indications should be strictly evaluated (evidence level IIb; recommendation B).
Ablative therapy for intrahepatic RHCC
The currently available loco-regional ablative techniques include radiofrequency ablation (RFA), microwave ablation (MWA), high-intensity focused ultrasound ablation (HIFU), cryotherapy (CRA) and percutaneous ethanol injection (PEI) (131-134).
Studies (114,135-137) have demonstrated that patients with RHCC after resection may benefit from RFA and even obtain comparable OS and DFS to those of patients undergoing repeat resection. Compared with repeat resection, the obvious advantages of ablation are minimal invasion, fewer complications and better repeatability. Additionally, the risk factors associated with recurrence and the interval of recurrence after the first operation can be neglected. The indications for RFA for RHCC patients are similar to those for primary HCC patients (138). For RHCC, the indications for RFA (139) are as follows: single tumor diameter ≤5 cm; tumor number ≤3 and maximum diameter ≤3 cm; absence of vessel tumor thrombus or invasion into adjacent organs; accessible ablation path evaluated by ultrasound scanning. Notably, for tumors with diameters >3 cm, overlapping modes of multiple ablations should be applied to complete ablation (140), or MWA could be performed (141-146).
Many studies have compared curative efficacy among CRA, RFA and MWA for treating HCC tumors <5 cm in diameter, but the differences in OS and DFS among groups were not significant. However, for tumors from 3 to 4 cm in diameter, local recurrence after CRA was lower than that after RFA (147,148). A study showed that HIFU has similar efficacy to RFA in treating RHCC meeting the Milan criteria (149). In addition, PEI could precisely be applied to ablate HCC ≤2 cm in diameter (150-153).
Recommendation 7
Patients with intrahepatic RHCC could benefit from ablation treatment. To avoid incomplete ablation or side effects, the appropriate ablation treatment should be carefully selected after preoperative evaluation, and standardized ablation procedures should be implemented (evidence level Ia; recommendation A).
Liver transplantation for intrahepatic RHCC
Many studies (154-157) have reported that salvage liver transplantation can provide survival benefit to patients with RHCC. Some studies (154,156) have even suggested that the OS was significantly better in patients with RHCC who underwent salvage liver transplantation than in patients who underwent repeat resection. In these studies, there were patients with HCC both within (155,157-159) and beyond (160,161) the Milan criteria before the initial operation; however, there was no major vascular tumor thrombus during the initial operation in the majority of patients. Most of the centers included in the studies required the tumors to meet the Milan criteria for the patients to be eligible for salvage liver transplantation (154,155,158,159,162); in addition, the recurrence interval after the initial operation could be >6 months or even >12 months. However, some centers adopted other criteria, such as the Kyoto criteria (163), the Kyushu University (KU) criteria (156), the Hangzhou criteria (160), and the up-to-seven criteria (161). Multiple studies (154,156,163) have also confirmed that living donor liver transplantation is safe and effective for RHCC patients. Nevertheless, there is a lack of comparative research on the prognosis of RHCC patients who have undergone deceased-donor liver transplantation or living donor liver transplantation.
Recommendation 8
Although patients with RHCC could benefit from liver transplantation, the RHCC must meet specific transplant criteria. Living donor liver transplantation could be equally safe and effective in salvage liver transplantation (evidence level, IIa; recommendation, B).
Surgical indications of resectable RHCC with macro vascular or bile duct invasion
There is still insufficient data on whether RHCC patients with macro vascular or bile duct invasion should undergo surgical resection. Further information may be presented in the updated version of this consensus in the future.
Indications of TACE for RHCC
Previous randomized controlled trials (RCTs) (164,165) have demonstrated that HCC patients with lesions localized within the liver without vascular invasion could benefit from TACE. Researches demonstrated that there was no superiority between TACE and bland embolization when treating HCC patients (166). Besides, Drug-eluting beads and conventional chemoembolization could also reach comparable results (167). Although TACE is not a radical treatment for HCC, approximately 10% of HCC patients achieve complete remission after receiving sequential TACE (168). In RHCC patients with ≤3 lesions and tumor diameter ≤5 cm, the effects of TACE and RFA may be comparable (169); in particular, for patients with multiple intrahepatic recurrences after resection or transplantation, TACE could effectively improve survival after recurrence (170). Investigators (107) have revealed that for RHCC within the Milan criteria, TACE and RFA/resection may exert the same effect on early RHCC, but RFA/resection may be better than TACE for late RHCC. Jin et al. (171) compared the effect of TACE, resection and RFA for RHCC with MVI. Their results indicated that TACE for MVI-positive RHCC patients resulted in better OS and DFS than did resection and RFA, especially in patients who experienced recurrence within 1 year of resection. Yang et al. (172) retrospectively analyzed the effect of TACE combined with RFA and TACE or RFA alone on RHCC and demonstrated that the 5-year survival was significantly higher in the combined treatment group than that in the TACE-only or the RFA-only group. Therefore, the indications for TACE are as follows (114,173): (I) presence of an RHCC lesion adjacent to an important vessel or bile duct preventing resection or ablation; (II) presence of multiple recurrent tumors within the liver; (III) incidence of early intrahepatic RHCC (within 1 year of resection); or (IV) patient’s willingness to receive TACE.
Recommendation 9
For patients with early intrahepatic RHCC (within 1 year after hepatectomy), if the lesion cannot be resected or ablated because of being adjacent to important vessels or bile duct and for patients with multiple RHCC lesions within the liver, TACE may control the progression of HCC and provide survival benefits (evidence, IIa; recommendation, A).
Radiotherapy for RHCC
Radiation therapy is one of the effective and safe therapeutic approaches for RHCC (174). Studies demonstrate that the 5-year survival rate for patients with HCC tumors ≤5 cm in diameter after radiotherapy can be more than 60% (175,176) and that the expected OS resulting from radiotherapy can be almost identical to that from RFA for HCC tumors <3 cm (177). However, there are only a few studies comparing the curative outcomes between radiotherapy and resection. Patients with RHCC may be ineligible for resection due to the location, size or number of tumors, large vascular tumor thrombosis, liver dysfunction or other factors. In addition, extrahepatic metastasis in the lung, bone and other organs is often observed in patients. Hence, appropriate radiotherapy could be repeatedly used to suppress tumor progression, alleviate disease symptoms and prolong patient survival. Studies have demonstrated that after radiotherapy, the 2-year survival rate of HCC patients with lung metastasis is 70.7%, and the median OS of HCC patients with bone metastasis is approximately 7.4 months (178-184). Additionally, the progression of RHCC can be effectively controlled in patients after liver transplantation. Radiotherapy can also be combined with other interventions to improve the prognosis of patients with advanced RHCC (185).
In several studies, patients with HCC who received TACE combined with doxorubicin-eluting beads (186) and radioactive microspheres (187) displayed high tumor necrosis rates and low progression rates. Nevertheless, the role of radiotherapy in treating RHCC remains to be further clarified.
Recommendation 10
Patients with RHCC tumors ≤5 cm in diameter that are not suitable for surgical resection could be treated with radiotherapy (evidence level, IIb; recommendation, B). Radiotherapy could also be used for RHCC patients with extrahepatic metastasis (evidence, III; recommendation, B).
General therapy for RHCC
Antiviral therapy
HBV can be reactivated by surgery, TACE or chemotherapy. Antiviral therapy can reduce the recurrence of HCC and improve patient survival after TACE and surgery. Therefore, for patients with HBV infection and active replication, antiviral therapy using oral nucleotide/nucleoside analogs is recommended (70,72).
Anti-tumor therapy
Molecular targeted drug
Sorafenib has been recognized as a molecular targeted drug for the treatment of advanced HCC. Two large international multi-center phase III trials have demonstrated that sorafenib confers survival benefit to advanced HCC patients in different countries, regions and with different underlying liver diseases (82). Additionally, regorafenib has been approved as a second-line molecular target for advanced HCC recently.
Systemic chemotherapy
Oxaliplatin has been approved for the treatment of HCC that is not suitable for resection or local treatment in China (188). The indications for this therapy are as follows: (I) advanced HCC with extrahepatic metastasis; (II) HCC lesions that are not suitable for surgical treatment or TACE; (III) HCC with tumor thrombus in the main portal vein or vena cava; (IV) vascular obstruction due to repeated TACE; and (V) recurrence after TACE treatment.
Immunotherapy
Immunotherapy for HCC includes immunomodulatory agents (thymosin α1, interferon α) (74,189), immune checkpoint inhibitors (e.g., CTLA-4 inhibitors, PD-1/PD-L1 inhibitors), tumor vaccine (such as dendritic cell vaccine) and cellular immunotherapy (190). The anti-tumor effects of these therapies need to be verified in large-scale clinical studies.
Palliative treatment
Moderate rehabilitation exercise, use of analgesics, improvement of sleep, increased nutrition, psychological therapy and other palliative treatment may enhance immunity and improve the quality of life and prognosis of patients.
Comprehensive treatment for recurrence after resection
In China, the management of HCC is multi-faceted and multidisciplinary. However, there is a contradiction between treatment approaches based on a hierarchical medical system and the implementation of well-organized and standardized HCC management system (3). Accordingly, the establishment of rational and standardized therapeutic options and comprehensive treatment for HCC under an MDT is extremely important, especially for the treatment of RHCC. Treatment with TACE, sorafenib and thymosin α1 in patients with a high risk of recurrence after resection, who have been treated with antiviral therapy, and treatment with RFA and TACE for patients with intrahepatic RHCC (172) are examples of combination therapies that improve survival.
Based on opinions demonstrated above, a decision-making path for RHCC treatment is presented in Figure 1. This decision-making path is according to the National Health and Family Planning Commission of the People’s Republic of China-Diagnosis, management, and treatment of HCC (V2017) (3).
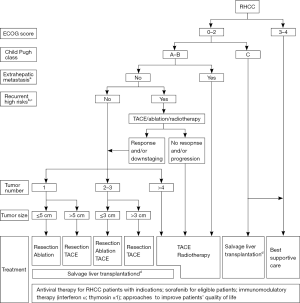
A treatment decision for a RHCC patient should be prudent. PS and liver function need to be considered in the first place. Characteristics of the initial HCC are important factors. TACE, ablation or radiotherapy should be attempted at first, if a RHCC patient with recurrent high-risk factors which are recurrence interval from initial resection to recurrence <1 year; presence of vascular invasion and/or multiple tumors in the initial HCC from operation findings or pathological reports. According to mRECIST criteria, if RHCC shows no response and/or progression after TACE/ablation/radiotherapy, radical therapies may not benefit for these patients. When RHCC presents response and/or downstaging after TACE/ablation/radiotherapy, treatment decision should be based on the number and size of the RHCC. Since there are few studies on the treatment for RHCC patients with PVTT (PVTT), it is difficult to provide a recommendation for these cases. Hence, we generally reach a consensus that patients with PVTT are recommended to be treated according to the Chinese Expert Consensus on multidisciplinary Diagnosis and Treatment of HCC with Portal Vein Tumor Thrombus (2016 edition) (35). For RHCC patients whose liver function show Child Pugh class C, if their ECOG score are 0–2 and within specific enlistment criteria for liver transplantation (e.g., Milan criteria, Hangzhou criteria, Kyoto criteria, Kyushu University criteria, Up-to-seven criteria, UCSF criteria, etc.), salvage liver transplantation could be considered. Otherwise, best supportive care should be given to these patients. Besides, general therapies should be taken into consideration during the whole process of the treatment for RHCC patients. General therapies include antiviral therapy for patients with indications, sorafenib for eligible patients, immunomodulatory therapy and any approach to improve patients’ quality of life.
Future perspectives
It is necessary to reiterate that this consensus for RHCC management is in its initial version, and thus the evidence from several studies may not be strong enough. For researchers, especially Chinese researchers, to achieve proper RHCC management a standardized guideline based on the situation in China is indispensable. Additionally, with emerging evidence and many related RCTs in progress, we hope that this initial version of the consensus can be further revised and validated.
The following aspects should be considered for the clinical management of RHCC: (I) An MDT is important for HCC management. Through a collaborative and effective MDT, great progress could be made in the prevention and treatment of RHCC after resection, thereby improving the overall prognostic outcomes of HCC patients; (II) the recurrence of HCC after resection prevents improvements in patient survival. The RFS and OS of patients are important indicators for the evaluation of curative effect. Efforts should also be made to improve the patients’ quality of life even when they are living with tumors; (3) the incidence of RHCC after resection is high in China and the conditions are complicated. Thus, there is a need to conduct RCTs and validate more effective methods of RHCC management. Additionally, the establishment of an RHCC sample database is necessary to discover the intrinsic molecular mechanisms of the occurrence and dissemination of RHCC. Furthermore, explorations of molecular classifications, targeted therapies and related translational treatment approaches for of HCC could potentially provide more evidence for the accurate treatment and prevention of HCC in China.
Acknowledgements
This article is supported by Chinese Society of Liver Cancer, Chinese Medical Doctor Association and Surgical Technology Innovation and Promotion Association, NAHIEM, China.
Funding: This work was in part supported by grants from the State Key Scientific and Technological Research Programs (2017ZX10203207-003-0020) and the Scientific and Technological Support Project of Sichuan Province (2018SZ0204, 2016SZ0025 and 2015SZ0049).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- National Health and Family Planning Commission of the People’s Republic of China. Diagnosis, management, and treatment of hepatocellular carcinoma (V2017). J Clin Hepatol 2017;33:1419-31. (In Chinese).
- Ryder SD. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (HCC) in adults. Gut 2003;52 Suppl 3:iii1-8. [Crossref] [PubMed]
- U.S. Preventive Services Task Force. Grade Definitions and Suggestions for Practice. 2012. Available online: http://www.uspreventiveservicestaskforce.org/Page/Name/grade-definitions
- Ministry of Health of the People's Republic of China. Diagnosis, management, and treatment of hepatocellular carcinoma (V2011). J Clin Hepatol 2011;27:1141-59. (in Chinese).
- European Association For The Study Of The Liver, European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908-43. [Crossref] [PubMed]
- Kudo M, Matsui O, Izumi N, et al. JSH Consensus-Based Clinical Practice Guidelines for the Management of Hepatocellular Carcinoma: 2014 Update by the Liver Cancer Study Group of Japan. Liver Cancer 2014;3:458-68. [Crossref] [PubMed]
- Kokudo N, Hasegawa K, Akahane M, et al. Evidence-based Clinical Practice Guidelines for Hepatocellular Carcinoma: The Japan Society of Hepatology 2013 update (3rd JSH-HCC Guidelines). Hepatol Res 2015;45. [Crossref] [PubMed]
- Verslype C, Rosmorduc O, Rougier P. Hepatocellular carcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23 Suppl 7:vii41-8. [Crossref] [PubMed]
- Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int 2017;11:317-70. [Crossref] [PubMed]
- Ahn SS, Kim MJ, Lim JS, et al. Added value of gadoxetic acid-enhanced hepatobiliary phase MR imaging in the diagnosis of hepatocellular carcinoma. Radiology 2010;255:459-66. [Crossref] [PubMed]
- Di Martino M, Marin D, Guerrisi A, et al. Intraindividual comparison of gadoxetate disodium-enhanced MR imaging and 64-section multidetector CT in the Detection of hepatocellular carcinoma in patients with cirrhosis. Radiology 2010;256:806-16. [Crossref] [PubMed]
- Golfieri R, Renzulli M, Lucidi V, et al. Contribution of the hepatobiliary phase of Gd-EOB-DTPA-enhanced MRI to Dynamic MRI in the detection of hypovascular small (≤ 2 cm) HCC in cirrhosis. Eur Radiol 2011;21:1233-42. [Crossref] [PubMed]
- Park MJ, Kim YK, Lee MW, et al. Small hepatocellular carcinomas: improved sensitivity by combining gadoxetic acid-enhanced and diffusion-weighted MR imaging patterns. Radiology 2012;264:761-70. [Crossref] [PubMed]
- Choi SH, Byun JH, Lim YS, et al. Diagnostic criteria for hepatocellular carcinoma 3 cm with hepatocyte-specific contrast-enhanced magnetic resonance imaging. J Hepatol 2016;64:1099-107. [Crossref] [PubMed]
- Mitchell DG, Bruix J, Sherman M, et al. LI-RADS (Liver Imaging Reporting and Data System): summary, discussion, and consensus of the LI-RADS Management Working Group and future directions. Hepatology 2015;61:1056-65. [Crossref] [PubMed]
- Best J, Bilgi H, Heider D, et al. The GALAD scoring algorithm based on AFP, AFP-L3, and DCP significantly improves detection of BCLC early stage hepatocellular carcinoma. Z Gastroenterol 2016;54:1296-305. [Crossref] [PubMed]
- Johnson PJ. The BALAD-2 and GALAD Biomarker Models for Hepatocellular Carcinoma. Gastroenterol Hepatol (N Y) 2017;13:231-3. [PubMed]
- Huang C, Zhu XD, Ji Y, et al. Microvascular invasion has limited clinical values in hepatocellular carcinoma patients at Barcelona Clinic Liver Cancer (BCLC) stages 0 or B. BMC Cancer 2017;17:58. [Crossref] [PubMed]
- Tribillon E, Barbier L, Goumard C, et al. When Should We Propose Liver Transplant After Resection of Hepatocellular Carcinoma? A Comparison of Salvage and De Principe Strategies. J Gastrointest Surg 2016;20:66-76; discussion 76. [Crossref] [PubMed]
- Section of Hepatic Surgery, Branch of Surgery, Chinese Medical Association. Expert consensus on selection of surgical treatments for hepatocellular carcinoma (2016 3rd edition). (in Chinese). Chin J Dig Surg 2017;16:113-5.
- Sala M, Fuster J, Llovet JM, et al. High pathological risk of recurrence after surgical resection for hepatocellular carcinoma: an indication for salvage liver transplantation. Liver Transpl 2004;10:1294-300. [Crossref] [PubMed]
- Shen JY, Li C, Wen TF, et al. Alpha fetoprotein changes predict hepatocellular carcinoma survival beyond the Milan criteria after hepatectomy. J Surg Res 2017;209:102-11. [Crossref] [PubMed]
- Liu X, Jiang H, Chen J, et al. Gadoxetic acid-enhanced MRI outperformed MDCT in diagnosing small hepatocellular carcinoma: A meta-analysis. Liver Transpl 2017;23:1505-18. [Crossref] [PubMed]
- Zheng SS, Xu X, Wu J, et al. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation 2008;85:1726-32. [Crossref] [PubMed]
- Shen JY, Li C, Wen TF, et al. Liver transplantation versus surgical resection for HCC meeting the Milan criteria: A propensity score analysis. Medicine (Baltimore) 2016;95. [Crossref] [PubMed]
- Kanai T, Hirohashi S, Upton MP, et al. Pathology of small hepatocellular carcinoma. A proposal for a new gross classification. Cancer 1987;60:810-9. [Crossref] [PubMed]
- Hui AM, Takayama T, Sano K, et al. Predictive value of gross classification of hepatocellular carcinoma on recurrence and survival after hepatectomy. J Hepatol 2000;33:975-9. [Crossref] [PubMed]
- Yang LY, Fang F, Ou DP, et al. Solitary large hepatocellular carcinoma: a specific subtype of hepatocellular carcinoma with good outcome after hepatic resection. Ann Surg 2009;249:118-23. [Crossref] [PubMed]
- Pawlik TM, Delman KA, Vauthey JN, et al. Tumor size predicts vascular invasion and histologic grade: Implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl 2005;11:1086-92. [Crossref] [PubMed]
- Cong WM, Bu H, Chen J, et al. Practice guidelines for the pathological diagnosis of primary liver cancer: 2015 update. World J Gastroenterol 2016;22:9279-87. [Crossref] [PubMed]
- Zhao H, Hua Y, Dai T, et al. Development and validation of a novel predictive scoring model for microvascular invasion in patients with hepatocellular carcinoma. Eur J Radiol 2017;88:32-40. [Crossref] [PubMed]
- Fan J, Zhou J, Wu ZQ, et al. Efficacy of different treatment strategies for hepatocellular carcinoma with portal vein tumor thrombosis. World J Gastroenterol 2005;11:1215-9. [Crossref] [PubMed]
- Cheng S, Chen M, Cai J. Chinese expert consensus on multidisciplinary diagnosis and treatment of hepatocellular carcinoma with portal vein tumor thrombus: 2016 edition. Oncotarget 2017;8:8867-76. [PubMed]
- Li J, Yan LN, Yang J, et al. Indicators of prognosis after liver transplantation in Chinese hepatocellular carcinoma patients. World J Gastroenterol 2009;15:4170-6. [Crossref] [PubMed]
- Li C, Liu JY, Peng W, et al. Liver resection versus transplantation for multiple hepatocellular carcinoma: a propensity score analysis. Oncotarget 2017;8:81492-500. [PubMed]
- Yang XR, Xu Y, Yu B, et al. High expression levels of putative hepatic stem/progenitor cell biomarkers related to tumour angiogenesis and poor prognosis of hepatocellular carcinoma. Gut 2010;59:953-62. [Crossref] [PubMed]
- Sun YF, Xu Y, Yang XR, et al. Circulating stem cell-like epithelial cell adhesion molecule-positive tumor cells indicate poor prognosis of hepatocellular carcinoma after curative resection. Hepatology 2013;57:1458-68. [Crossref] [PubMed]
- Kiriyama S, Uchiyama K, Ueno M, et al. Triple positive tumor markers for hepatocellular carcinoma are useful predictors of poor survival. Ann Surg 2011;254:984-91. [Crossref] [PubMed]
- Shirabe K, Toshima T, Kimura K, et al. New scoring system for prediction of microvascular invasion in patients with hepatocellular carcinoma. Liver Int 2014;34:937-41. [Crossref] [PubMed]
- Liu CL, Fan ST, Cheung ST, et al. Anterior approach versus conventional approach right hepatic resection for large hepatocellular carcinoma: a prospective randomized controlled study. Ann Surg 2006;244:194-203. [Crossref] [PubMed]
- Man K, Fan ST, Ng IO, et al. Prospective evaluation of Pringle maneuver in hepatectomy for liver tumors by a randomized study. Ann Surg 1997;226:704-11; discussion 711-3. [Crossref] [PubMed]
- Wen T, Chen Z, Yan L, et al. Continuous normothermic hemihepatic vascular inflow occlusion over 60 min for hepatectomy in patients with cirrhosis caused by hepatitis B virus. Hepatol Res 2007;37:346-52. [Crossref] [PubMed]
- Giuliante F, Ardito F, Pulitano C, et al. Does hepatic pedicle clamping affect disease-free survival following liver resection for colorectal metastases? Ann Surg 2010;252:1020-6. [Crossref] [PubMed]
- Yang T, Zhang J, Lu JH, et al. Risk factors influencing postoperative outcomes of major hepatic resection of hepatocellular carcinoma for patients with underlying liver diseases. World J Surg 2011;35:2073-82. [Crossref] [PubMed]
- Lu Q, Luo Y, Yuan CX, et al. Value of contrast-enhanced intraoperative ultrasound for cirrhotic patients with hepatocellular carcinoma: a report of 20 cases. World J Gastroenterol 2008;14:4005-10. [Crossref] [PubMed]
- Zhang T, Zeng Y, Huang J, et al. Combined resection with radiofrequency ablation for bilobar hepatocellular carcinoma: a single-center experience. J Surg Res 2014;191:370-8. [Crossref] [PubMed]
- Shindoh J, Makuuchi M, Matsuyama Y, et al. Complete removal of the tumor-bearing portal territory decreases local tumor recurrence and improves disease-specific survival of patients with hepatocellular carcinoma. J Hepatol 2016;64:594-600. [Crossref] [PubMed]
- Shi M, Guo RP, Lin XJ, et al. Partial hepatectomy with wide versus narrow resection margin for solitary hepatocellular carcinoma: a prospective randomized trial. Ann Surg 2007;245:36-43. [Crossref] [PubMed]
- Nara S, Shimada K, Sakamoto Y, et al. Prognostic impact of marginal resection for patients with solitary hepatocellular carcinoma: evidence from 570 hepatectomies. Surgery 2012;151:526-36. [Crossref] [PubMed]
- Wu H, Lu Q, Luo Y, et al. Application of contrast-enhanced intraoperative ultrasonography in the decision-making about hepatocellular carcinoma operation. World J Gastroenterol 2010;16:508-12. [Crossref] [PubMed]
- Shen JY, Li C, Wen TF, et al. A simple prognostic score system predicts the prognosis of solitary large hepatocellular carcinoma following hepatectomy. Medicine (Baltimore) 2016;95. [Crossref] [PubMed]
- Eguchi S, Kanematsu T, Arii S, et al. Comparison of the outcomes between an anatomical subsegmentectomy and a non-anatomical minor hepatectomy for single hepatocellular carcinomas based on a Japanese nationwide survey. Surgery 2008;143:469-75. [Crossref] [PubMed]
- Poon RT, Fan ST, Ng IO, et al. Significance of resection margin in hepatectomy for hepatocellular carcinoma: A critical reappraisal. Ann Surg 2000;231:544-51. [Crossref] [PubMed]
- Makino Y, Yamanoi A, Kimoto T, et al. The influence of perioperative blood transfusion on intrahepatic recurrence after curative resection of hepatocellular carcinoma. Am J Gastroenterol 2000;95:1294-300. [Crossref] [PubMed]
- Buczkowski AK, Kim PT, Ho SG, et al. Multidisciplinary management of ruptured hepatocellular carcinoma. J Gastrointest Surg 2006;10:379-86. [Crossref] [PubMed]
- Kim BW, Kim YB, Wang HJ, et al. Risk factors for immediate post-operative fatal recurrence after curative resection of hepatocellular carcinoma. World J Gastroenterol 2006;12:99-104. [Crossref] [PubMed]
- Zhu WJ, Huang CY, Li C, et al. Risk factors for early recurrence of HBV-related hepatocellular carcinoma meeting milan criteria after curative resection. Asian Pac J Cancer Prev 2013;14:7101-6. [Crossref] [PubMed]
- Lee CW, Chan KM, Lee CF, et al. Hepatic resection for hepatocellular carcinoma with lymph node metastasis: clinicopathological analysis and survival outcome. Asian J Surg 2011;34:53-62. [Crossref] [PubMed]
- Nobuoka D, Kato Y, Gotohda N, et al. Postoperative serum alpha-fetoprotein level is a useful predictor of recurrence after hepatectomy for hepatocellular carcinoma. Oncol Rep 2010;24:521-8. [PubMed]
- Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020-2. [Crossref] [PubMed]
- Ren ZG, Lin ZY, Xia JL, et al. Postoperative adjuvant arterial chemoembolization improves survival of hepatocellular carcinoma patients with risk factors for residual tumor: a retrospective control study. World J Gastroenterol 2004;10:2791-4. [Crossref] [PubMed]
- Zhong JH, Li H, Li LQ, et al. Adjuvant therapy options following curative treatment of hepatocellular carcinoma: a systematic review of randomized trials. Eur J Surg Oncol 2012;38:286-95. [Crossref] [PubMed]
- Lai EC, Lo CM, Fan ST, et al. Postoperative adjuvant chemotherapy after curative resection of hepatocellular carcinoma: a randomized controlled trial. Arch Surg 1998;133:183-8. [Crossref] [PubMed]
- Ke-Wei L, Tian-Fu W, Xi L, et al. The effect of postoperative TACE on prognosis of HCC with microscopic venous invasion. Hepatogastroenterology 2012;59:1944-6. [PubMed]
- Lau WY, Leung TW, Ho SK, et al. Adjuvant intra-arterial iodine-131-labelled lipiodol for resectable hepatocellular carcinoma: a prospective randomised trial. Lancet 1999;353:797-801. [Crossref] [PubMed]
- Peng BG, He Q, Li JP, et al. Adjuvant transcatheter arterial chemoembolization improves efficacy of hepatectomy for patients with hepatocellular carcinoma and portal vein tumor thrombus. Am J Surg 2009;198:313-8. [Crossref] [PubMed]
- Zhong JH, Li LQ. Postoperative adjuvant transarterial chemoembolization for participants with hepatocellular carcinoma: A meta-analysis. Hepatol Res 2010;40:943-53. [Crossref] [PubMed]
- Huang G, Lau WY, Wang ZG, et al. Antiviral therapy improves postoperative survival in patients with hepatocellular carcinoma: a randomized controlled trial. Ann Surg 2015;261:56-66. [Crossref] [PubMed]
- Wong JS, Wong GL, Tsoi KK, et al. Meta-analysis: the efficacy of anti-viral therapy in prevention of recurrence after curative treatment of chronic hepatitis B-related hepatocellular carcinoma. Aliment Pharmacol Ther 2011;33:1104-12. [Crossref] [PubMed]
- Yin J, Li N, Han Y, et al. Effect of antiviral treatment with nucleotide/nucleoside analogs on postoperative prognosis of hepatitis B virus-related hepatocellular carcinoma: a two-stage longitudinal clinical study. J Clin Oncol 2013;31:3647-55. [Crossref] [PubMed]
- Gish RG, Gordon SC, Nelson D, et al. A randomized controlled trial of thymalfasin plus transarterial chemoembolization for unresectable hepatocellular carcinoma. Hepatol Int 2009;3:480-9. [Crossref] [PubMed]
- He C, Peng W, Li C, et al. Thymalfasin, a promising adjuvant therapy in small hepatocellular carcinoma after liver resection. Medicine (Baltimore) 2017;96. [Crossref] [PubMed]
- Cheng SQ, Wu MC, Chen H, et al. Transcatheter hepatic arterial chemoembolization and thymosin alpha1 in postoperative treatment of hepatocellular carcinoma. Zhonghua Zhong Liu Za Zhi 2004;26:305-7. (in Chinese). [PubMed]
- Breitenstein S, Dimitroulis D, Petrowsky H, et al. Systematic review and meta-analysis of interferon after curative treatment of hepatocellular carcinoma in patients with viral hepatitis. Br J Surg 2009;96:975-81. [Crossref] [PubMed]
- Ikeda K, Arase Y, Saitoh S, et al. Interferon beta prevents recurrence of hepatocellular carcinoma after complete resection or ablation of the primary tumor-A prospective randomized study of hepatitis C virus-related liver cancer. Hepatology 2000;32:228-32. [Crossref] [PubMed]
- Lo CM, Liu CL, Chan SC, et al. A randomized, controlled trial of postoperative adjuvant interferon therapy after resection of hepatocellular carcinoma. Ann Surg 2007;245:831-42. [Crossref] [PubMed]
- Takayama T, Sekine T, Makuuchi M, et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet 2000;356:802-7. [Crossref] [PubMed]
- Bruix J, Takayama T, Mazzaferro V, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol 2015;16:1344-54. [Crossref] [PubMed]
- Pressiani T, Boni C, Rimassa L, et al. Sorafenib in patients with Child-Pugh class A and B advanced hepatocellular carcinoma: a prospective feasibility analysis. Ann Oncol 2013;24:406-11. [Crossref] [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [Crossref] [PubMed]
- Kakizaki S, Sohara N, Sato K, et al. Preventive effects of vitamin K on recurrent disease in patients with hepatocellular carcinoma arising from hepatitis C viral infection. J Gastroenterol Hepatol 2007;22:518-22. [Crossref] [PubMed]
- Mizuta T, Ozaki I, Eguchi Y, et al. The effect of menatetrenone, a vitamin K2 analog, on disease recurrence and survival in patients with hepatocellular carcinoma after curative treatment: a pilot study. Cancer 2006;106:867-72. [Crossref] [PubMed]
- Okita K, Izumi N, Matsui O, et al. Peretinoin after curative therapy of hepatitis C-related hepatocellular carcinoma: a randomized double-blind placebo-controlled study. J Gastroenterol 2015;50:191-202. [Crossref] [PubMed]
- Yoshida H, Shiratori Y, Kudo M, et al. Effect of vitamin K2 on the recurrence of hepatocellular carcinoma. Hepatology 2011;54:532-40. [Crossref] [PubMed]
- Muto Y, Moriwaki H, Ninomiya M, et al. Prevention of second primary tumors by an acyclic retinoid, polyprenoic acid, in patients with hepatocellular carcinoma. Hepatoma Prevention Study Group. N Engl J Med 1996;334:1561-7. [Crossref] [PubMed]
- Hasegawa K, Takayama T, Ijichi M, et al. Uracil-tegafur as an adjuvant for hepatocellular carcinoma: a randomized trial. Hepatology 2006;44:891-5. [Crossref] [PubMed]
- Xia Y, Qiu Y, Li J, et al. Adjuvant therapy with capecitabine postpones recurrence of hepatocellular carcinoma after curative resection: a randomized controlled trial. Ann Surg Oncol 2010;17:3137-44. [Crossref] [PubMed]
- Poon RT, Fan ST, Ng IO, et al. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer 2000;89:500-7. [Crossref] [PubMed]
- Sakamoto M, Hirohashi S, Tsuda H, et al. Multicentric independent development of hepatocellular carcinoma revealed by analysis of hepatitis B virus integration pattern. Am J Surg Pathol 1989;13:1064-7. [Crossref] [PubMed]
- The general rules for the clinical and pathological study of primary liver cancer. Liver Cancer Study Group of Japan. Jpn J Surg 1989;19:98-129. [Crossref] [PubMed]
- Takenaka K, Adachi E, Nishizaki T, et al. Possible multicentric occurrence of hepatocellular carcinoma: a clinicopathological study. Hepatology 1994;19:889-94. [Crossref] [PubMed]
- Ng IO, Guan XY, Poon RT, et al. Determination of the molecular relationship between multiple tumour nodules in hepatocellular carcinoma differentiates multicentric origin from intrahepatic metastasis. J Pathol 2003;199:345-53. [Crossref] [PubMed]
- Chen PJ, Chen DS, Lai MY, et al. Clonal origin of recurrent hepatocellular carcinomas. Gastroenterology 1989;96:527-9. [Crossref] [PubMed]
- Hodges KB, Cummings OW, Saxena R, et al. Clonal origin of multifocal hepatocellular carcinoma. Cancer 2010;116:4078-85. [Crossref] [PubMed]
- Wang B, Xia CY, Lau WY, et al. Determination of clonal origin of recurrent hepatocellular carcinoma for personalized therapy and outcomes evaluation: a new strategy for hepatic surgery. J Am Coll Surg 2013;217:1054-62. [Crossref] [PubMed]
- Morimoto O, Nagano H, Sakon M, et al. Diagnosis of intrahepatic metastasis and multicentric carcinogenesis by microsatellite loss of heterozygosity in patients with multiple and recurrent hepatocellular carcinomas. J Hepatol 2003;39:215-21. [Crossref] [PubMed]
- Esumi M, Aritaka T, Arii M, et al. Clonal origin of human hepatoma determined by integration of hepatitis B virus DNA. Cancer Res 1986;46:5767-71. [PubMed]
- Iizuka N, Oka M, Yamada-Okabe H, et al. Oligonucleotide microarray for prediction of early intrahepatic recurrence of hepatocellular carcinoma after curative resection. Lancet 2003;361:923-9. [Crossref] [PubMed]
- Cheung ST, Chen X, Guan XY, et al. Identify metastasis-associated genes in hepatocellular carcinoma through clonality delineation for multinodular tumor. Cancer Res 2002;62:4711-21. [PubMed]
- Barry CT, D'Souza M, McCall M, et al. Micro RNA expression profiles as adjunctive data to assess the risk of hepatocellular carcinoma recurrence after liver transplantation. Am J Transplant 2012;12:428-37. [Crossref] [PubMed]
- Wilkens L, Bredt M, Flemming P, et al. Differentiation of multicentric origin from intra-organ metastatic spread of hepatocellular carcinomas by comparative genomic hybridization. J Pathol 2000;192:43-51. [Crossref] [PubMed]
- Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 2012;366:883-92. [Crossref] [PubMed]
- Miao R, Luo H, Zhou H, et al. Identification of prognostic biomarkers in hepatitis B virus-related hepatocellular carcinoma and stratification by integrative multi-omics analysis. J Hepatol 2014;61:840-9. [Crossref] [PubMed]
- Meniconi RL, Komatsu S, Perdigao F, et al. Recurrent hepatocellular carcinoma: a Western strategy that emphasizes the impact of pathologic profile of the first resection. Surgery 2015;157:454-62. [Crossref] [PubMed]
- Zhang X, Li C, Wen T, et al. Appropriate treatment strategies for intrahepatic recurrence after curative resection of hepatocellular carcinoma initially within the Milan criteria: according to the recurrence pattern. Eur J Gastroenterol Hepatol 2015;27:933-40. [Crossref] [PubMed]
- Wang DY, Liu L, Qi XS, et al. Hepatic Re-resection Versus Transarterial Chemoembolization for the Treatment of Recurrent Hepatocellular Carcinoma after Initial Resection: a Systematic Review and Meta-analysis. Asian Pac J Cancer Prev 2015;16:5573-8. [Crossref] [PubMed]
- Mise Y, Hasegawa K, Shindoh J, et al. The Feasibility of Third or More Repeat Hepatectomy for Recurrent Hepatocellular Carcinoma. Ann Surg 2015;262:347-57. [Crossref] [PubMed]
- Yamashita Y, Shirabe K, Tsuijita E, et al. Third or more repeat hepatectomy for recurrent hepatocellular carcinoma. Surgery 2013;154:1038-45. [Crossref] [PubMed]
- Wu CC, Cheng SB, Yeh DC, et al. Second and third hepatectomies for recurrent hepatocellular carcinoma are justified. Br J Surg 2009;96:1049-57. [Crossref] [PubMed]
- Poon RT, Fan ST, O'Suilleabhain CB, et al. Aggressive management of patients with extrahepatic and intrahepatic recurrences of hepatocellular carcinoma by combined resection and locoregional therapy. J Am Coll Surg 2002;195:311-8. [Crossref] [PubMed]
- Minagawa M, Makuuchi M, Takayama T, et al. Selection criteria for repeat hepatectomy in patients with recurrent hepatocellular carcinoma. Ann Surg 2003;238:703-10. [Crossref] [PubMed]
- Wang K, Liu G, Li J, et al. Early intrahepatic recurrence of hepatocellular carcinoma after hepatectomy treated with re-hepatectomy, ablation or chemoembolization: a prospective cohort study. Eur J Surg Oncol 2015;41:236-42. [Crossref] [PubMed]
- Zou Q, Li J, Wu D, et al. Nomograms for Pre-operative and Post-operative Prediction of Long-Term Survival of Patients Who Underwent Repeat Hepatectomy for Recurrent Hepatocellular Carcinoma. Ann Surg Oncol 2016;23:2618-26. [Crossref] [PubMed]
- Yamashita S, Aoki T, Inoue Y, et al. Outcome of salvage hepatic resection for recurrent hepatocellular carcinoma after radiofrequency ablation therapy. Surgery 2015;157:463-72. [Crossref] [PubMed]
- Nakajima Y, Ko S, Kanamura T, et al. Repeat liver resection for hepatocellular carcinoma. J Am Coll Surg 2001;192:339-44. [Crossref] [PubMed]
- Dong JH, Zheng SS, Chen XP, et al. Consensus on evaluation of Hepatic functional reserve before hepatectomy(2011 edition) Chinese Journal of Digestive Surgery 2011;10:20-25. (in Chinese).
- Kubota K, Makuuchi M, Kusaka K, et al. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology 1997;26:1176-81. [PubMed]
- Shirabe K, Shimada M, Gion T, et al. Postoperative liver failure after major hepatic resection for hepatocellular carcinoma in the modern era with special reference to remnant liver volume. J Am Coll Surg 1999;188:304-9. [Crossref] [PubMed]
- Shindoh J, D, Tzeng CW, Vauthey JN. Portal vein embolization for hepatocellular carcinoma. Liver Cancer 2012;1:159-67. [Crossref] [PubMed]
- Siriwardana RC, Lo CM, Chan SC, et al. Role of portal vein embolization in hepatocellular carcinoma management and its effect on recurrence: a case-control study. World J Surg 2012;36:1640-6. [Crossref] [PubMed]
- Tsujita E, Utsunomiya T, Ohta M, et al. Outcome of repeat hepatectomy in patients with hepatocellular carcinoma aged 75 years and older. Surgery 2010;147:696-703. [Crossref] [PubMed]
- Sposito C, Battiston C, Facciorusso A, et al. Propensity score analysis of outcomes following laparoscopic or open liver resection for hepatocellular carcinoma. Br J Surg 2016;103:871-80. [Crossref] [PubMed]
- Liu K, Chen Y, Wu X, et al. Laparoscopic liver re-resection is feasible for patients with posthepatectomy hepatocellular carcinoma recurrence: a propensity score matching study. Surg Endosc 2017;31:4790-8. [Crossref] [PubMed]
- Chan AC, Poon RT, Chok KS, et al. Feasibility of laparoscopic re-resection for patients with recurrent hepatocellular carcinoma. World J Surg 2014;38:1141-6. [Crossref] [PubMed]
- Hu M, Zhao G, Xu D, et al. Laparoscopic repeat resection of recurrent hepatocellular carcinoma. World J Surg 2011;35:648-55. [Crossref] [PubMed]
- Zhang J, Zhou ZG, Huang ZX, et al. Prospective, single-center cohort study analyzing the efficacy of complete laparoscopic resection on recurrent hepatocellular carcinoma. Chin J Cancer 2016;35:25. [Crossref] [PubMed]
- Kanazawa A, Tsukamoto T, Shimizu S, et al. Laparoscopic liver resection for treating recurrent hepatocellular carcinoma. J Hepatobiliary Pancreat Sci 2013;20:512-7. [Crossref] [PubMed]
- Belli G, Cioffi L, Fantini C, et al. Laparoscopic redo surgery for recurrent hepatocellular carcinoma in cirrhotic patients: feasibility, safety, and results. Surg Endosc 2009;23:1807-11. [Crossref] [PubMed]
- Lu MD, Yin XY, Xie XY, et al. Percutaneous thermal ablation for recurrent hepatocellular carcinoma after hepatectomy. Br J Surg 2005;92:1393-8. [Crossref] [PubMed]
- Chen HW, Lai EC, Zhen ZJ, et al. Ultrasound-guided percutaneous cryotherapy of hepatocellular carcinoma. Int J Surg 2011;9:188-91. [Crossref] [PubMed]
- Thomasset SC, Dennison AR, Garcea G. Ablation for recurrent hepatocellular carcinoma: a systematic review of clinical efficacy and prognostic factors. World J Surg 2015;39:1150-60. [Crossref] [PubMed]
- Facciorusso A, Serviddio G, Muscatiello N. Local ablative treatments for hepatocellular carcinoma: An updated review. World J Gastrointest Pharmacol Ther 2016;7:477-89. [Crossref] [PubMed]
- Chen R, Gan Y, Ge N, et al. Transarterial Chemoembolization versus Radiofrequency Ablation for Recurrent Hepatocellular Carcinoma after Resection within Barcelona Clinic Liver Cancer Stage 0/A: A Retrospective Comparative Study. J Vasc Interv Radiol 2016;27:1829-36. [Crossref] [PubMed]
- Chan AC, Poon RT, Cheung TT, et al. Survival analysis of re-resection versus radiofrequency ablation for intrahepatic recurrence after hepatectomy for hepatocellular carcinoma. World J Surg 2012;36:151-6. [Crossref] [PubMed]
- Ho CM, Lee PH, Shau WY, et al. Survival in patients with recurrent hepatocellular carcinoma after primary hepatectomy: comparative effectiveness of treatment modalities. Surgery 2012;151:700-9. [Crossref] [PubMed]
- Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg 2006;243:321-8. [Crossref] [PubMed]
- Liang HH, Chen MS, Peng ZW, et al. Percutaneous radiofrequency ablation versus repeat hepatectomy for recurrent hepatocellular carcinoma: a retrospective study. Ann Surg Oncol 2008;15:3484-93. [Crossref] [PubMed]
- Chen MH, Yang W, Yan K, et al. Large liver tumors: protocol for radiofrequency ablation and its clinical application in 110 patients--mathematic model, overlapping mode, and electrode placement process. Radiology 2004;232:260-71. [Crossref] [PubMed]
- Zhang L, Wang N, Shen Q, et al. Therapeutic efficacy of percutaneous radiofrequency ablation versus microwave ablation for hepatocellular carcinoma. PLoS One 2013;8. [Crossref] [PubMed]
- Abdelaziz A, Elbaz T, Shousha HI, et al. Efficacy and survival analysis of percutaneous radiofrequency versus microwave ablation for hepatocellular carcinoma: an Egyptian multidisciplinary clinic experience. Surg Endosc 2014;28:3429-34. [Crossref] [PubMed]
- Lencioni R, Crocetti L. Local-regional treatment of hepatocellular carcinoma. Radiology 2012;262:43-58. [Crossref] [PubMed]
- Farina L, Weiss N, Nissenbaum Y, et al. Characterisation of tissue shrinkage during microwave thermal ablation. Int J Hyperthermia 2014;30:419-28. [Crossref] [PubMed]
- Facciorusso A, Di Maso M, Muscatiello N. Microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma: A systematic review and meta-analysis. Int J Hyperthermia 2016;32:339-44. [Crossref] [PubMed]
- Luo W, Zhang Y, He G, et al. Effects of radiofrequency ablation versus other ablating techniques on hepatocellular carcinomas: a systematic review and meta-analysis. World J Surg Oncol 2017;15:126. [Crossref] [PubMed]
- Ei S, Hibi T, Tanabe M, et al. Cryoablation provides superior local control of primary hepatocellular carcinomas of >2 cm compared with radiofrequency ablation and microwave coagulation therapy: an underestimated tool in the toolbox. Ann Surg Oncol 2015;22:1294-300. [Crossref] [PubMed]
- Wang C, Wang H, Yang W, et al. Multicenter randomized controlled trial of percutaneous cryoablation versus radiofrequency ablation in hepatocellular carcinoma. Hepatology 2015;61:1579-90. [Crossref] [PubMed]
- Chan AC, Cheung TT, Fan ST, et al. Survival analysis of high-intensity focused ultrasound therapy versus radiofrequency ablation in the treatment of recurrent hepatocellular carcinoma. Ann Surg 2013;257:686-92. [Crossref] [PubMed]
- Giorgio A, Di Sarno A, De Stefano G, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma compared to percutaneous ethanol injection in treatment of cirrhotic patients: an Italian randomized controlled trial. Anticancer Res 2011;31:2291-5. [PubMed]
- Brunello F, Veltri A, Carucci P, et al. Radiofrequency ablation versus ethanol injection for early hepatocellular carcinoma: A randomized controlled trial. Scand J Gastroenterol 2008;43:727-35. [Crossref] [PubMed]
- Shiina S, Teratani T, Obi S, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology 2005;129:122-30. [Crossref] [PubMed]
- Lencioni RA, Allgaier HP, Cioni D, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology 2003;228:235-40. [Crossref] [PubMed]
- Yong CC, Tsai MC, Lin CC, et al. Comparison of Salvage Living Donor Liver Transplantation and Local Regional Therapy for Recurrent Hepatocellular Carcinoma. World J Surg 2016;40:2472-80. [Crossref] [PubMed]
- Chan AC, Chan SC, Chok KS, et al. Treatment strategy for recurrent hepatocellular carcinoma: salvage transplantation, repeated resection, or radiofrequency ablation? Liver Transpl 2013;19:411-9. [Crossref] [PubMed]
- Yamashita Y, Yoshida Y, Kurihara T, et al. Surgical results for recurrent hepatocellular carcinoma after curative hepatectomy: Repeat hepatectomy versus salvage living donor liver transplantation. Liver Transpl 2015;21:961-8. [Crossref] [PubMed]
- Del Gaudio M, Ercolani G, Ravaioli M, et al. Liver transplantation for recurrent hepatocellular carcinoma on cirrhosis after liver resection: University of Bologna experience. Am J Transplant 2008;8:1177-85. [Crossref] [PubMed]
- Lee HS, Choi GH, Joo DJ, et al. The clinical behavior of transplantable recurrent hepatocellular carcinoma after curative resection: implications for salvage liver transplantation. Ann Surg Oncol 2014;21:2717-24. [Crossref] [PubMed]
- Majno PE, Sarasin FP, Mentha G, et al. Primary liver resection and salvage transplantation or primary liver transplantation in patients with single, small hepatocellular carcinoma and preserved liver function: an outcome-oriented decision analysis. Hepatology 2000;31:899-906. [Crossref] [PubMed]
- Hu Z, Zhou J, Li Z, et al. Time interval to recurrence as a predictor of overall survival in salvage liver transplantation for patients with hepatocellular carcinoma associated with hepatitis B virus. Surgery 2015;157:239-48. [Crossref] [PubMed]
- Guerrini GP, Gerunda GE, Montalti R, et al. Results of salvage liver transplantation. Liver Int 2014;34:e96-e104. [Crossref] [PubMed]
- Hu RH, Ho MC, Wu YM, et al. Feasibility of salvage liver transplantation for patients with recurrent hepatocellular carcinoma. Clin Transplant 2005;19:175-80. [Crossref] [PubMed]
- Kaido T, Mori A, Ogura Y, et al. Living donor liver transplantation for recurrent hepatocellular carcinoma after liver resection. Surgery 2012;151:55-60. [Crossref] [PubMed]
- Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 2002;359:1734-9. [Crossref] [PubMed]
- Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 2002;35:1164-71. [Crossref] [PubMed]
- Facciorusso A, Bellanti F, Villani R, et al. Transarterial chemoembolization vs bland embolization in hepatocellular carcinoma: A meta-analysis of randomized trials. United European Gastroenterol J 2017;5:511-8. [Crossref] [PubMed]
- Facciorusso A, Di Maso M, Muscatiello N. Drug-eluting beads versus conventional chemoembolization for the treatment of unresectable hepatocellular carcinoma: A meta-analysis. Dig Liver Dis 2016;48:571-7. [Crossref] [PubMed]
- Jin YJ, Chung YH, Kim JA, et al. Predisposing factors of hepatocellular carcinoma recurrence following complete remission in response to transarterial chemoembolization. Dig Dis Sci 2013;58:1758-65. [Crossref] [PubMed]
- Koh PS, Chan AC, Cheung TT, et al. Efficacy of radiofrequency ablation compared with transarterial chemoembolization for the treatment of recurrent hepatocellular carcinoma: a comparative survival analysis. HPB (Oxford) 2016;18:72-8. [Crossref] [PubMed]
- Cheng YC, Chen TW, Fan HL, et al. Transarterial chemoembolization for intrahepatic multiple recurrent HCC after liver resection or transplantation. Ann Transplant 2014;19:309-16. [Crossref] [PubMed]
- Jin YJ, Lee JW, Lee OH, et al. Transarterial chemoembolization versus surgery/radiofrequency ablation for recurrent hepatocellular carcinoma with or without microvascular invasion. J Gastroenterol Hepatol 2014;29:1056-64. [Crossref] [PubMed]
- Yang W, Chen MH, Wang MQ, et al. Combination therapy of radiofrequency ablation and transarterial chemoembolization in recurrent hepatocellular carcinoma after hepatectomy compared with single treatment. Hepatol Res 2009;39:231-40. [Crossref] [PubMed]
- Dai WC, Cheung TT. Strategic overview on the best treatment option for intrahepaitc hepatocellular carcinoma recurrence. Expert Rev Anticancer Ther 2016;16:1063-72. [Crossref] [PubMed]
- Bae SH, Park HC, Lim DH, et al. Salvage treatment with hypofractionated radiotherapy in patients with recurrent small hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2012;82:e603-7. [Crossref] [PubMed]
- Su TS, Liang P, Lu HZ, et al. Stereotactic body radiation therapy for small primary or recurrent hepatocellular carcinoma in 132 Chinese patients. J Surg Oncol 2016;113:181-7. [Crossref] [PubMed]
- Sanuki N, Takeda A, Oku Y, et al. Stereotactic body radiotherapy for small hepatocellular carcinoma: a retrospective outcome analysis in 185 patients. Acta Oncol 2014;53:399-404. [Crossref] [PubMed]
- Seo YS, Kim MS, Yoo HJ, et al. Radiofrequency ablation versus stereotactic body radiotherapy for small hepatocellular carcinoma: a Markov model-based analysis. Cancer Med 2016;5:3094-101. [Crossref] [PubMed]
- Nakazawa T, Adachi S, Kitano M, et al. Potential prognostic benefits of radiotherapy as an initial treatment for patients with unresectable advanced hepatocellular carcinoma with invasion to intrahepatic large vessels. Oncology 2007;73:90-7. [Crossref] [PubMed]
- Otsuka M, Ohara K, Takada Y, et al. Radiation therapy for intrahepatic recurrence after hepatectomy for hepatocellular carcinoma. Int J Clin Oncol 2003;8:151-5. [Crossref] [PubMed]
- Huang WY, Jen YM, Lee MS, et al. Stereotactic body radiation therapy in recurrent hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2012;84:355-61. [Crossref] [PubMed]
- He J, Zeng ZC, Tang ZY, et al. Clinical features and prognostic factors in patients with bone metastases from hepatocellular carcinoma receiving external beam radiotherapy. Cancer 2009;115:2710-20. [Crossref] [PubMed]
- Chang UK, Kim MS, Han CJ, et al. Clinical result of stereotactic radiosurgery for spinal metastasis from hepatocellular carcinoma: comparison with conventional radiation therapy. J Neurooncol 2014;119:141-8. [Crossref] [PubMed]
- Komatsu S, Fukumoto T, Demizu Y, et al. The effectiveness of particle radiotherapy for hepatocellular carcinoma associated with inferior vena cava tumor thrombus. J Gastroenterol 2011;46:913-20. [Crossref] [PubMed]
- Rivera L, Giap H, Miller W, et al. Hepatic intra-arterial infusion of yttrium-90 microspheres in the treatment of recurrent hepatocellular carcinoma after liver transplantation: a case report. World J Gastroenterol 2006;12:5729-32. [Crossref] [PubMed]
- Oh D, Lim DH, Park HC, et al. Early three-dimensional conformal radiotherapy for patients with unresectable hepatocellular carcinoma after incomplete transcatheter arterial chemoembolization: a prospective evaluation of efficacy and toxicity. Am J Clin Oncol 2010;33:370-5. [Crossref] [PubMed]
- Song MJ, Chun HJ, Song DS, et al. Comparative study between doxorubicin-eluting beads and conventional transarterial chemoembolization for treatment of hepatocellular carcinoma. J Hepatol 2012;57:1244-50. [Crossref] [PubMed]
- Salem R, Gordon AC, Mouli S, et al. Y90 Radioembolization Significantly Prolongs Time to Progression Compared With Chemoembolization in Patients With Hepatocellular Carcinoma. Gastroenterology 2016;151:1155-63.e2. [Crossref] [PubMed]
- Qin S, Bai Y, Lim HY, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol 2013;31:3501-8. [Crossref] [PubMed]
- Sun HC, Tang ZY, Wang L, et al. Postoperative interferon alpha treatment postponed recurrence and improved overall survival in patients after curative resection of HBV-related hepatocellular carcinoma: a randomized clinical trial. J Cancer Res Clin Oncol 2006;132:458-65. [Crossref] [PubMed]
- Xu L, Wang J, Kim Y, et al. A randomized controlled trial on patients with or without adjuvant autologous cytokine-induced killer cells after curative resection for hepatocellular carcinoma. Oncoimmunology 2015;5. [Crossref] [PubMed]


