Inspiration of liver resection for hepatocellular carcinoma associated with hepatic vein invasion, not inferior vena cava invasion
The treatment for advanced hepatocellular carcinoma (HCC) has been limited to transarterial chemoembolization (TACE) or medical therapy as a palliative treatment. Patients with HCC that grossly invades the portal or hepatic vein have a limited life span. Thus, the current Barcelona Clinic for Liver Cancer (BCLC) staging system recommends systemic therapy with sorafenib (1). However, several papers (2-5) have reported their results of TACE, hepatic resection, or transplantation in patients with macroscopic vascular invasion (MVI), especially portal vein tumor thrombus (PVTT). The treatment for HCC with MVI is still under debate.
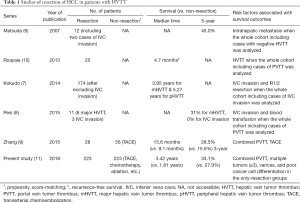
Meanwhile, because of the rarity of hepatic vein tumor thrombus (HVTT), compared to PTTV, only a few papers (6-9) have reported surgical outcomes focused on HVTT (Table 1). Several other studies (12,13) dealt with the surgical outcomes of patients with HCC and PVTT and/or inferior vena cava tumor thrombus (IVCTT), as well as HVTT. PVTT and IVCTT are well known as comprising aggressive tumor biology and resulting in poor outcomes. Thus, the analysis that focused on the prognosis of surgical outcomes of patients with HCC and HVTT was difficult in these studies.
Full table
Four years ago, Kokudo et al. (7) conducted a single-center study of surgical resection of HCC in patients with HVTT. In their report, they showed excellent outcomes of 153 patients resulting in a 90-day mortality rate of 5% and a median survival time of 5.27 years. The risk factors for overall survival were IVCTT and R1/2 resection. However, it was a retrospective single-arm study, and the cohort was small.
Recently, the same coauthors, Kokudo et al. (11), retrospectively analyzed the impact of hepatic resection in Child-Pugh class A patients with HCC and HVTT through Japanese nationwide survey. This time, the authors wisely solved several issues raised in the previous study: a case-control study via a 1:1 propensity-matching nonsurgical control group, collecting a large cohort number via a nationwide survey considering the rarity of HVTT. They proved again the excellent survival outcomes in the resection group compared to those in the non-resection group; the median survival time after resection was longer than that after nonsurgical treatment (3.42 vs. 1.81 years, retrospectively) and a 90-day mortality rate of 3.4%. In both the resection and non-resection groups, high-risk patients for hepatic resection were included: those who were elderly (median age, 66.3 vs. 67.5 years), exhibited portal hypertension (gastroesophageal varices, 13.5% vs. 11.7%), and were accompanied PVTT (40.4% vs. 40.4%). In addition, they suggested the risk factors that are associated with poor overall survival after resection: combined PVTT, multiple tumors (≥3), varices, and poor cancer cell differentiation. In this study, they excluded patients with IVCTT, which had been a risk factor of survival in the previous study (7), from a comparison between the surgical and nonsurgical control groups and from multivariate risk factor analysis of the resection group. Therefore, resection of these tumors with IVCTT may not be justified, given the poor outcomes and a high 90-day mortality rate.
Although this study inspirited clinicians who manage these patients with advanced HCC and HVTT, several unanswered, critical questions are as follows. First, this study was based on a nationwide survey, rather than on concrete data. For this reason, they could not provide detailed surgical data, as well as tips and pitfalls of resection, like en bloc resection, total exclusion/Pringle maneuver, or pulmonary protection against tumor embolus. Second, the definition of the presence of MVI was determined by the radiologic findings. In addition, they did not report pathologic differentiation of tumors, even though poor cancer cell differentiation was one of the risk factors for overall survival. Third, this study represented the Japanese characteristics of HCC, of which more than half of the original liver diseases were hepatitis C-related. This study might follow the previous version of Japanese regulations for HCC (14), in which the indications of surgery and other nonsurgical treatment may be different from those in Western and other Asian countries. Finally, the period of this study was before sorafenib application in Japan. For this reason, a future study regarding the comparison between outcomes of hepatic resection and sorafenib for HCC and HVTT is expected.
This study suggested a great deal of hope for patients with advanced HCC involving HVTT, as well as for clinicians who manage these patients, although the results were probably affected by selection bias for better tumor biology and less difficult situations (location and reserved liver function) for surgery. Recent development of novel immunotherapies and/or target therapies may be helpful in the future (15), by adding neoadjuvant and/or adjuvant therapy to improve recurrence-free survival rates in patients who exhibit successful local control by liver resection, but show systemic recurrence or intrahepatic metastasis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Bruix J, Raoul JL, Sherman M, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol 2012;57:821-9. [Crossref] [PubMed]
- Kim KM, Kim JH, Park IS, et al. Reappraisal of repeated transarterial chemoembolization in the treatment of hepatocellular carcinoma with portal vein invasion. J Gastroenterol Hepatol 2009;24:806-14. [Crossref] [PubMed]
- Chung JW, Park JH, Han JK, et al. Hepatocellular carcinoma and portal vein invasion: results of treatment with transcatheter oily chemoembolization. AJR Am J Roentgenol 1995;165:315-21. [Crossref] [PubMed]
- Kokudo T, Hasegawa K, Matsuyama Y, et al. Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J Hepatol 2016;65:938-43. [Crossref] [PubMed]
- Lee KW, Suh SW, Choi Y, et al. Macrovascular invasion is not an absolute contraindication for living donor liver transplantation. Liver Transpl 2017;23:19-27. [Crossref] [PubMed]
- Matsuda M, Suzuki T, Kono H, et al. Predictors of hepatic venous trunk invasion and prognostic factors in patients with hepatocellular carcinomas that had come into contact with the trunk of major hepatic veins. J Hepatobiliary Pancreat Surg 2007;14:289-96. [Crossref] [PubMed]
- Kokudo T, Hasegawa K, Yamamoto S, et al. Surgical treatment of hepatocellular carcinoma associated with hepatic vein tumor thrombosis. J Hepatol 2014;61:583-8. [Crossref] [PubMed]
- Pesi B, Ferrero A, Grazi GL, et al. Liver resection with thrombectomy as a treatment of hepatocellular carcinoma with major vascular invasion: results from a retrospective multicentric study. Am J Surg 2015;210:35-44. [Crossref] [PubMed]
- Zhang YF, Wei W, Guo ZX, et al. Hepatic resection versus transcatheter arterial chemoembolization for the treatment of hepatocellular carcinoma with hepatic vein tumor thrombus. Jpn J Clin Oncol 2015;45:837-43. [Crossref] [PubMed]
- Roayaie S, Jibara G, Taouli B, et al. Resection of hepatocellular carcinoma with macroscopic vascular invasion. Ann Surg Oncol 2013;20:3754-60. [Crossref] [PubMed]
- Kokudo T, Hasegawa K, Matsuyama Y, et al. Liver resection for hepatocellular carcinoma associated with hepatic vein invasion: A Japanese nationwide survey. Hepatology 2017;66:510-7. [Crossref] [PubMed]
- Pawlik TM, Poon RT, Abdalla EK, et al. Hepatectomy for hepatocellular carcinoma with major portal or hepatic vein invasion: results of a multicenter study. Surgery 2005;137:403-10. [Crossref] [PubMed]
- Shaohua L, Qiaoxuan W, Peng S, et al. Surgical Strategy for Hepatocellular Carcinoma Patients with Portal/Hepatic Vein Tumor Thrombosis. PLoS One 2015;10. [Crossref] [PubMed]
- Kokudo N, Hasegawa K, Akahane M, et al. Evidence-based Clinical Practice Guidelines for Hepatocellular Carcinoma: The Japan Society of Hepatology 2013 update (3rd JSH-HCC Guidelines). Hepatol Res 2015;45. [Crossref] [PubMed]
- da Motta Girardi D, Correa TS, Crosara Teixeira M, et al. Hepatocellular Carcinoma: Review of Targeted and Immune Therapies. J Gastrointest Cancer 2018;49:227-36. [Crossref] [PubMed]


