Complete portal vein occlusion after cyanoacrylate sclerotherapy in biliary atresia treated by living donor liver transplantation with intraoperative portal vein stenting via segment 4 portal vein stump
Introduction
Living donor liver transplantation (LDLT) is a durable treatment for end-stage liver disease due to biliary atresia (BA) with excellent graft and patient survivals as shown in our previous study (1). Vascular complications are a major cause of graft failure in LDLT, particularly in children, where size disparity between donor and recipient vessels is often unavoidable. The risk of portal vein (PV) thrombosis is augmented in cases with pre-transplant PV pathological change, which is frequently seen in BA with periportal inflammation and fibrosis from recurrent cholangitis. In these cases, surgical reconstruction can be mired with technical difficulties due to hypoplastic, sclerotic PV. Thus, it is no surprise that PV complications are the most common surgical complications in pediatric LDLT (1,2).
Our group previously identified PV size <4 mm and PV flow <10 cm/sec are independent risk factors for intra-operative PV complications (3), and combined with hepatic artery resistance index <0.65, are strong warning signs that predict the development of post-transplant PV complications (4). For these patients, primary reconstruction of the suboptimal PV often fails to restore sufficient blood flow. Fortunately, advances in endovascular techniques in managing PV complications have evolved over the past decade, with percutaneous transhepatic and transjugular approaches now standard in the post-operative period (3,5). Experience gained from these techniques has allowed extension to intra-operative approaches via the inferior mesenteric vein (IMV) and segment 4 PV stump (P4 stump). The intra-operative PV stent obviates the need for further treatment during the tenuous post-transplant period, and can successfully sustain long-term portal flow (6). The authors have previously described their novel innovative approaches (7) and a series of PV stenting via the P4 stump route (8), but this is the most challenging and the first instance that this technique was performed in a case with extensive thromboses of PV, spleno-mesenteric junction and splenic vein, and trans-mesocolic tunneled IMV was used as an alternate source for portal inflow reconstruction.
Case presentation
The patient was a fourteen-month old girl (weight =8.1 kg; height =67.3 cm) with decompensated liver disease secondary to BA (total bilirubin 22.9 mg/dL; INR 1.75; albumin 2.8 gm/dL; child class C; CTP 10; PELD 23) who was referred to our center from overseas. Despite early diagnosis of BA at 10 weeks of age, Kasai hepatoportojejunostomy was not undertaken. Over the subsequent months, the child developed deepening jaundice and recurrent variceal bleeding, warranting multiple hospitalizations for blood and albumin transfusions, and a total of three endoscopic sclerotherapy procedures (the first at 6 months of age) prior to transplantation.
A Doppler ultrasound 2 weeks after the first sclerotherapy showed a patent main PV that measured 4.6 mm in diameter from a previous size of 6.1 mm. There was flow reversal and dampened phasicity without thrombus. Further assessment with computed tomography angiogram showed the hepatic arterial, venous, and portal systems were patent. The main PV measured 5 mm but the intrahepatic portion of the PV was not clearly demonstrated. The donor was the patient’s aunt who was evaluated to be acceptable to donate the left lateral segment (LLS).
In preparation for LDLT at our center, repeat computed tomography angiogram showed extensive thrombosis of esophagogastric varices with retrograde extension causing total occlusion of main PV, spleno-mesenteric junction and splenic vein following cyanoacrylate sclerotherapy. The IMV was elongated and engorged (Figure 1). Despite the PV findings, the child was still deemed eligible for LDLT with the IMV considered as a possible alternate source for PV inflow reconstruction.
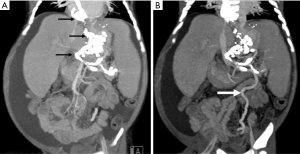
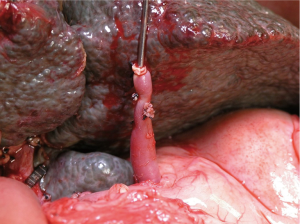
The child underwent LDLT (LLS graft weight 243 gm; GSLV 82.3%; GRWR 2.76%). Where total occlusion of the PV extending down to the spleno-mesenteric junction was confirmed. The IMV was found to be elongated and engorged with a diameter of 4 mm and mean pressure of 33.67 mmHg. The IMV was hydrostatically dilated and tunneled through the mesocolon for reconstruction with the graft PV (Figure 2). The graft PV, measured 8 mm, was anastomosed to the trans-mesocolic tunneled IMV using 7-0 PDS suture. The graft hepatic vein was reconstructed with the recipient inferior vena cava triple venoplasty (9). The arterial and biliary reconstructions were both done by microsurgical techniques (10).
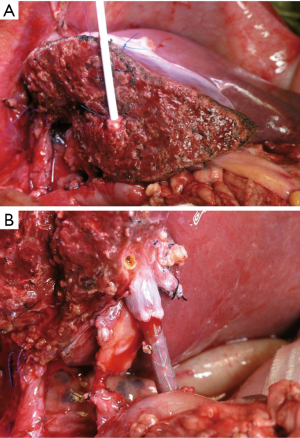
Upon completion of vascular anastomoses, the PV flow was 9.8 cm/sec. Due to low PV flow and small IMV size, intra-operative PV stenting was decided with the goal of augmenting flow by increasing the IMV diameter and correcting the angulation of the trans-mesocolic tunneled IMV. The ligated P4 stump in the cut surface of the graft was re-opened. A 7-French introducer sheath (Terumo, Tokyo, Japan) was inserted into the P4 stump (Figure 3A). An initial contrast portography to serve as “road map” was done. A 0.018 and 0.035-inch guide wire followed by a 4-French J curve catheter (Terumo) were used to traverse the PV anastomosis. As the guide wire was advanced, repeat contrast studies were obtained as necessary. Two 10-mm expandable metallic stents with lengths of 60 and 40 mm (Bard E Luminexx Vascular Stent, Boston Scientific, Natick, MA, USA) were deployed (Figure 3B). After stenting, the access orifice was closed by ligating the P4 stump again. The IMV size increased from 4 to 6.7 mm. The PV flow increased to 20.2 cm/sec after deployment of the two stents. The child was placed on prophylactic anti-platelets as protocol. She recovered uneventfully without complications and is back in Abu Dhabi doing well now 3 years after LDLT.
Discussion
Sclerotherapy is an effective way to bridge esophago-gastric varices bleeding while awaiting liver transplantation. A caveat is when the varices are located in the fundal region of the stomach that is difficult to access despite advances in flexible endoscopy. There are different materials used for sclerotherapy. Cyanoacrylate (Krazy glue, Super glue, methyl-2-cyanoacrylate, ethyl-2-cyanoacrylate) is a fast adhesive glue that has been used medically. Its strong rapid-acting adhesiveness and irritant quality make it an ideal choice sclerosant. A problem related with sclerosing agents when they are injected directly into the veins is the possibility of embolization. This observation is seen during sclerotherapy for peripheral varicose veins in the extremities involving varicosities >3 mm. Distal digital compression on the vein prevents embolic complications during sclerotherapy of varicose veins. This digital compression maneuver is impractical during injection sclerotherapy of bleeding esophago-gastric varices.
Injection sclerotherapy of large esophago-gastric varices may be difficult to achieve without injecting a sizeable amount of sclerosing agent into the varices. Considering that these veins have multiple communicating channels, the injected sclerosant may spill into the origin of these collaterals. In the authors’ case the thrombosis extended beyond the esophago-gastric varices propagating extensively into PV, spleno-mesenteric junction and splenic vein. It is known that downstream splenic vein ligation and its collaterals may cause further lowering of the PV flow in an already sluggish portal circulation in liver cirrhosis. Retrograde spilling of sclerosant and sluggish PV flow may be a plausible explanation for the aggravation to complete PV thrombosis.
The use of intra-operative portography is helpful as a road map for PV reconstruction. In the case described, it would not be advisable to perform the portogram through the superior mesenteric vein (SMV) or the transsplenic approach due to spleno-mesenteric junction and splenic vein occlusion (Figure 1). Although the introducer sheath and stent may be inserted through the IMV, it may not be prudent to manipulate the thin and fragile textured IMV as it can precipitate injury and later thrombosis. Compared to the IMV, the P4 stump is thicker, already exposed, and easy to manipulate. Hence, the option to re-open the P4 stump and access the PV-IMV anastomosis through this route is preferable. It is seldom possible to harvest a long fresh autologous interposition vein graft to bridge the patent SMV and graft PV, whereas readily available cryopreserved vascular allograft or synthetic graft is famed for premature failure. The use of vascular stents to correct size disparity and acute angulation of the trans-mesocolic tunneled IMV may prove as a more effective way of securing adequate portal inflow. Vascular stenting is a less invasive endovascular technique which scores over more technically demanding surgical revision with benefits of durable patency at no additional warm ischemia (5). Although the SMV was patent, unsuccessful manipulations through it will further jeopardize the channels and risk the patient with mesenteric ischemia. From our experience, key components that lead to a successful outcome of this approach are: the sturdy, larger graft P4 stump, short access route and optimal angulation (8). Another essential component of our intra-operative stenting approach is the use of self-expandable metallic stents. Due to their flexibility, these devices not only increase the caliber of the PV, but can also correct anastomotic angulation and act as a scaffold to maintain the luminal diameter after deployment into the PV, ensuring sufficient flow (Figure 3B).
Graft P4 stump approach for stenting is a viable option for children with limited options for reconstruction in the presence of complete PV occlusion. Stenting appears to be a better option than a technically demanding exercise of interposition vein graft. This report also highlighted the possible complications that may arise following cyanoacrylate sclerotherapy for bleeding esophago-gastric varices.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Chen CL, Concejero AM, Wang CC, et al. Living donor liver transplantation with biliary atresia: Single center experience with first 100 cases. Am J Transplant 2006;6:2672. [Crossref] [PubMed]
- Ueda M, Oike F, Kasahara M, et al. Portal vein complication in pediatric living donor liver transplantation using left-side grafts. Am J Transplant 2008;8:2097. [Crossref] [PubMed]
- Cheng YF, Ou HY, Yu CY, et al. Section 8. Management of portal venous complications in pediatric living donor liver transplantation. Transplantation 2014;97 Suppl 8:S32-4. [Crossref] [PubMed]
- Ou HY, Concejero AM, Huang TL, et al. Portal vein thrombosis in biliary atresia patients after living donor liver transplantation. Surgery 2011;149:40. [Crossref] [PubMed]
- Cheng YF, Ou HY, Tsang LLC, et al. Vascular stents in the management of portal venous complications in living donor liver transplantation. Am J Transplant 2010;10:1276. [Crossref] [PubMed]
- Kim YJ, Ko GY, Yoon HK, et al. Intraoperative stent placement in the portal vein during or after liver transplantation. Liver Transpl 2007;13:1145. [Crossref] [PubMed]
- Chen CL, Concejero AM, Ou HY, et al. Intraoperative portal vein stent placement in pediatric living donor liver transplantation. J Vasc Interv Radiol 2012;23:724. [Crossref] [PubMed]
- Chen CL, Cheng YF, Huang V, et al. P4 stump approach for intraoperative portal vein stenting in pediatric living donor liver transplantation: An innovative technique for a challenging problem. Ann Surg 2018;267. [Crossref] [PubMed]
- de Villa VH, Chen CL, Chen YS, et al. Outflow tract reconstruction in living donor liver transplantation. Transplantation 2000;70:1604. [Crossref] [PubMed]
- Lin TS, Concejero AM, Chen CL, et al. Routine microsurgical biliary reconstruction decreases early anastomotic complications in living donor liver transplantation. Liver Transpl 2009;15:1766. [Crossref] [PubMed]


