Cohort contributions to trends in the incidence and mortality of intrahepatic cholangiocarcinoma
Introduction
Cholangiocarcinoma is the second most common primary liver cancer and its incidence and mortality are increasing worldwide (1-3). Cholangiocarcinoma, which arises from the epithelium of the bile duct, can be categorized as intrahepatic or extrahepatic (2,4). Intrahepatic cholangiocarcinoma (ICC) has an aggressive tumor biology with a mortality to incidence ratio of 0.95 and a 5-year survival of less than 20% (5,6). In addition, hospitalizations for cholangiocarcinoma and hospital charges per patient have increased since the late 1990s (7). Due to the fatal and costly nature of cholangiocarcinoma, a coordinated multidisciplinary approach to patient care is recommended. In turn, understanding trends and contributory mechanisms in ICC incidence and mortality may be important for managing the disease at the population level (8).
In the United States, reported ICC has increased from 0.44 to 1.18 cases per 100,000 people between 1973 and 2012 (9). Some portion of the reported increase in incidence may be secondary to misclassification of perihilar (Klatskin) extrahepatic cholangiocarcinomas (ECC) as ICC (10-12). However, in examining the impact of birth cohort on ICC incidence, it has been noted that in most countries including the United States, ICC incidence has plateaued in recent generations (13). Mortality rates for ICC have also been increasing in the United States, from 2.2 per 100,000 to 3.0 per 100,000 between 1999 and 2014 (14,15). The effect of birth cohort on ICC mortality has not been well defined. In fact, the overall contribution of cohort effects to contemporary trends in ICC incidence and mortality remains uncertain. As such, the objective of the current study was to use age-period-cohort (APC) modeling to evaluate the contribution of cohort effects on trends in ICC incidence and mortality in the United States.
Methods
The study was deemed exempt from review by the Institutional Review Board of The Ohio State University. Incidence data and corresponding population data were acquired from the Surveillance, Epidemiology and End-Results (SEER) program (16). Incidence and population data for 1973–2013 were obtained from nine registries (Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound and Utah). Patients with ICC were identified using the International Classification of Diseases (ICD)-O-2/ICD-O-3 topography. Primary site code C22.1 (intrahepatic bile duct carcinoma), histologic type codes 8160 (bile duct adenocarcinoma), and behavior code 3 (mortality) were used (17,18). Patients with extrahepatic cholangiocarcinoma and patients with mixed hepatocholangiocarcinoma were specifically excluded from consideration, as these disease entities have a distinct biology, treatment approach, as well as epidemiology.
The incidence data were grouped according to 5-year intervals of age at diagnosis or “Age” (indexed by the first year, e.g., 50 referring to ages 50–54 years); year of diagnosis or “Period” (indexed by the midpoint year, e.g., 1980 referring to 1978–1982); and year of birth or “Cohort” (indexed by the midpoint year). Age-adjusted incidence rates of ICC included birth cohorts ranging from 1900 to 1970, age groups ranging from 50 to 75, and periods from 1975 to 2010. ICC mortality data were obtained from the Centers for Disease Control and Prevention National Center for Health Statistics WONDER Online Multiple Cause of Death Database 1999–2015. This database includes national mortality and population data based on death certificates for United States residents. Deaths were included in the analysis according to International Classification of Diseases-10th revision (ICD-10) codes. Deaths which were coded as having an underlying cause (single or multiple) of ICC (code C22.1, Intrahepatic cholangiocarcinoma) were included in the analysis. Data were extracted for six 5-year age groups, indexed by the first year (e.g., 50 representing 50–54 years old), spanning ages 50–84.
For APC modeling of ICC mortality, the year of death was considered as the period; three 5-year periods indexed by the first year (e.g., 2000 representing 2000–2004) were selected for analysis spanning the years 2000–2014. The period indexed by the year 2000 served as the reference. Birth cohorts were determined by subtracting age in years from year of death and were grouped into ten 5-year cohorts indexed by the first year (e.g., 1915 representing 1915–1919) spanning birth years 1915–1965. The 1940 cohort was used as the reference.
APC models using restricted cubic splines (Stata package apcfit) were fitted to characterize the contribution of cohort effect to ICC incidence and mortality, as previously described (19,20). In the APC modeling framework, the incidence of an event (ICC diagnosis or death due to ICC) was modeled as a count using Poisson regression. APC models included independent variables for age (age at diagnosis), period (year of diagnosis for incidence and year of death for mortality), and cohort (year of birth). The exponentiated regression coefficients on these parameters produced incidence risk ratios (IRRs) that described differences in risk (e.g., risk of ICC diagnosis) according to age, period or cohort. In contemporary applications, the APC model has been refined by treating age, period, and cohort as continuous variables modeled as a sequence of cubic functions (i.e., modeled using restricted cubic splines), which allows estimating APC influences on cancer incidence or mortality as smooth functions without requiring strong assumptions about their form (19,20). For incidence, APC modeling was performed for the entire cohort due to limited coverage of the SEER Registry. For mortality, gender-specific APC modeling was performed.
According to recommended presentation of data from these models, age-specific rates were plotted graphically in one pane (on the left), while IRRs of cohort and period were plotted in another pane (on the right), to illustrate modification of age-specific incidence based on period and cohort (19,20). These APC models were compared to age-period models, not including cohort, using a likelihood ratio test to determine whether accounting for cohort differences achieved statistically significant improvement in model fit (21). The impact of the cohort term in APC modeling can be assessed by omitting the cohort term, which is the same as constraining the cohort effect to null. A likelihood ratio test comparing the age-period model to the unconstrained APC model, summarizes whether the APC model is an improvement over the modeling the impact of age and period alone (21). Predicted relative incidence and mortality rates were obtained with the 1940 birth cohort and 1990 period set as the reference categories. Data analysis was performed using Stata/MP 14.2 (College Station, TX, USA: StataCorp, LP).
Results
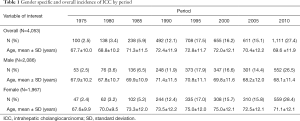
There were 4,053 ICC cases ICC diagnosed at SEER registry sites between 1973 and 2012. Overall, among patients diagnosed with ICC, 2,086 (51.5%) were male and 1,967 (48.5%) were female. Over the period indexed as 1975 (i.e., spanning years 1973–1977), there were 100 (2.5%) cases diagnosed with a mean patient age of 67.7±10.0 years. In comparison, during the period indexed as 2010, the number of diagnosed ICC increased to 1,111 (27.4%) with a mean patient age of 69.6±11.9 years. In all intervening time periods except for 2000 and 2005, there was an increase in the number of ICC cases diagnosed with a rising number of ICC cases among both female and male patients. Characteristics of patients diagnosed with ICC are summarized by period in Table 1.
Full table
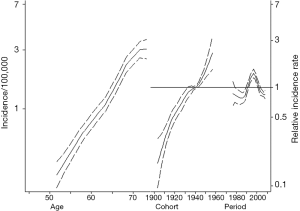
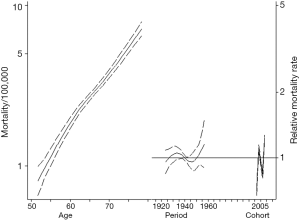
Figure 1 summarizes the APC model for ICC incidence. ICC incidence increased with older age. Specifically, among patients aged 70–75 years, the incidence of ICC was 3.0/100,000 compared with only 1.1/100,000 for those patients aged 60–65 years. ICC incidence also increased across all birth cohorts examined. In fact, each successive cohort had an increase in the IRR for ICC versus the reference cohort (1945 IRR: 1.08, 95% CI: 1.02–1.15; 1950 IRR: 1.34, 95% CI: 1.18–1.53; 1955 IRR: 1.80, 95% CI: 1.38–2.34). In comparing the APC models to age-period models, the inclusion of cohort effects tended to improve the model fit relative to an age-period model (likelihood ratio test P=0.082).
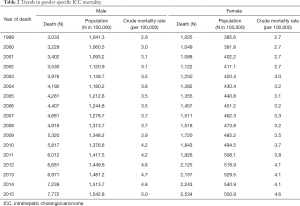
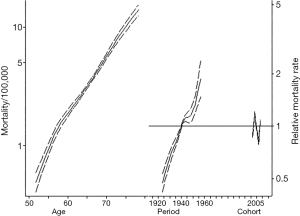
Table 2 demonstrates trends in gender-specific ICC mortality. Among males, crude mortality increased from 2.9 per 100,000 in 1999 to 5.0 per 100,000 in 2015. Among females the crude mortality incidence increased from 2.7 per 100,000 in 1999 to 4.6 per 100,000 in 2015. Figure 2 demonstrates age, period, and cohort effects on mortality of ICC in the United Sates among United States males. In comparing the APC model to an age-period model, the addition of the cohort effect improved the fit of the model over a model including age and period alone (likelihood ratio test P=0.001). In addition, each successive cohort had an increase in IRR for incidence of mortality (1925 IRR: 0.47, 95% CI: 0.42–0.53; 1930 IRR: 0.61, 95% CI: 0.55–0.67; 1935 IRR: 0.75, 95% CI: 0.70–0.81; 1945 IRR: 1.11, 95% CI: 1.07–1.17; 1950 IRR: 1.15, 95% CI: 1.05–1.27; 1955 IRR: 1.46, 95% CI: 1.27–1.68). Figure 3 demonstrates age, period, and cohort effects on mortality of ICC among US females. In contrast to male patients, in comparing the APC model to an age-period model, the addition of cohort did not improve the model over a model including age and period alone (likelihood ratio test P=0.223). An increase in IRRs for incidence of mortality was noted, however, over time with an IRR of 0.96 (95% CI: 0.84–1.08) in 1935 versus an IRR of 1.15 (95% CI: 0.90–1.48) in 1955.
Full table
Discussion
Cholangiocarcinoma incidence and mortality are increasing worldwide (1-3). In this study, we used APC modeling to evaluate the impact of birth cohort effects on ICC incidence and mortality. Of note, each successive cohort saw an increase in ICC incidence and the overall contribution of birth cohort effects tended to impact the trends in ICC incidence (likelihood ratio test P=0.082). One important finding of the current study was the finding that, among both males and females, the crude ICC-related mortality rate nearly doubled between 1999 and 2015. Interestingly, ICC-related mortality risk varied across birth cohorts in United States males. In contrast, ICC-related mortality risk did not vary across birth cohorts among females.
In a previous study, Petrick et al. examined the incidence of primary liver cancer in various countries between 1978 and 2007 using the Cancer Incidence in Five Continents electronic database. In that study, the authors reported an increasing incidence of ICC in the United States with a 4.2% average annual percentage change. Additionally, using APC modeling to explore the impact of cohort effect on ICC incidence, the authors reported that ICC incidence rates had plateaued with successive cohorts (6). In contrast, using data with a longer follow-up data from the SEER 9 sites, the current study did not demonstrate a plateauing of ICC incidence. Rather, the incidence of ICC increased in the most recent birth cohorts. In addition, the likelihood ratio test suggested that broader trends in ICC incidence could have been summarized equally well by modeling ICC incidence as a function of age and period alone. An important finding in the current study was that ICC-related mortality risk varied across birth cohorts among males, yet not across birth cohorts for females. The reason for the sex-based difference in the birth cohort ICC mortality rate among men versus women is likely multifactorial. The sex difference in cancer susceptibility is a consistent finding in cancer epidemiology and may be attributable to hormonal or behavioral differences (22). To this point, there may be variations in the different exposures of each birth cohort (e.g., hepatitis C, diabetes, etc.) among men versus women that contributed to the sex-based birth cohort differences in ICC incidence.
While increasing incidence and mortality in HCC can be explained by changes in risk factors such as metabolic syndrome, obesity, diabetes, and hepatitis C, no clear explanation exists for changes in ICC incidence and mortality (6). Improvements in diagnostic modalities were initially hypothesized to account, in part, for the increasing incidence (23). There has not been, however, an increase in the identification of early or small tumors detected over time (24). The increased incidence in ICC has also been attributed to possible changes in the classification system of ICC versus ECC. Specifically, previous misclassification due to inadequate histopathological techniques may have inappropriately skewed the classification of cholangiocarcinoma as carcinomas not otherwise specified (NOS) (10-12). Furthermore, the increasing incidence in ICC has been attributed to a rising incidence of hepatitis C, as well as possibly steatosis (25-27). Additionally, as the APC model did not dramatically improve fit over an age-period model, it is possible that the increasing trend in ICC incidence is related to the continuing aging of the population.
The current study had several strengths and limitations. Mortality data were obtained from the Center for Disease Control and Prevention National Center for Health Statistics WONDER Online Database. These data included validated, county-level national mortality and population data based on death certificates for US residents. The underlying cause of death was entered by the physician on the death certification and was not necessarily the true underlying cause of death. For the analysis of ICC incidence, the SEER database was used. The SEER database has inherent limitations including no available data on the etiology of primary liver disease, as well as the risk factors for liver disease such as hepatitis B and C status, alcoholic cirrhosis or non-alcoholic steatohepatitis. Data on the specific different types of treatment utilized to treat ICC were also not available. While some level of misclassification may have been possible, the pathological diagnostic validity of the SEER database has been previously validated (28). In addition, the SEER 9 database has been deemed to be generally representative of the US population, yet the data may not be representative of cancer incidence in rural areas (28).
In conclusion, ICC incidence and mortality have increased over time in the United States. APC modeling tended to improve the model fit relative to an age-period model regarding ICC incidence and mortality. Although ICC incidence risk increased over successive birth cohorts, the addition of birth cohort effects did not dramatically improve the ability to account for trends in ICC incidence versus modeling trends as products of only age and period. In the case of mortality, birth cohort was related to ICC mortality risk among men, but not among women. In turn, ICC mortality risk may be driven by gender-specific risk factors and the increasing incidence of ICC in older patients. Further investigation into the mechanism behind increasing ICC incidence and mortality is needed to help identify potential areas for intervention on a population level.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was deemed exempt from review by the Institutional Review Board of The Ohio State University.
References
- Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology 2001;33:1353-7. [Crossref] [PubMed]
- Rahnemai-Azar AA, Weisbrod A, Dillhoff M, et al. Intrahepatic cholangiocarcinoma: Molecular markers for diagnosis and prognosis. Surg Oncol 2017;26:125-37. [Crossref] [PubMed]
- Fitzmaurice C, Allen C, Barber RM, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2017;3:524-48. [Crossref] [PubMed]
- Brown KM, Parmar AD, Geller DA. Intrahepatic Cholangiocarcinoma. Surg Oncol Clin N Am 2014;23:231-46. [Crossref] [PubMed]
- Guro H, Kim JW, Choi Y, et al. Multidisciplinary management of intrahepatic cholangiocarcinoma: Current approaches. Surg Oncol 2017;26:146-52. [Crossref] [PubMed]
- Petrick JL, Braunlin M, Laversanne M, et al. International trends in liver cancer incidence, overall and by histologic subtype, 1978-2007. Int J Cancer 2016;139:1534-45. [Crossref] [PubMed]
- Wadhwa V, Jobanputra Y, Thota PN, et al. Healthcare utilization and costs associated with cholangiocarcinoma. Gastroenterol Rep (Oxf) 2017;5:213-8. [PubMed]
- Weber SM, Ribero D, O'Reilly EM, et al. Intrahepatic cholangiocarcinoma: expert consensus statement. HPB (Oxford) 2015;17:669-80. [Crossref] [PubMed]
- Saha SK, Zhu AX, Fuchs CS, et al. Forty-Year Trends in Cholangiocarcinoma Incidence in the U.S.: Intrahepatic Disease on the Rise. Oncologist 2016;21:594-9. [Crossref] [PubMed]
- Welzel TM, McGlynn KA, Hsing AW, et al. Impact of classification of hilar cholangiocarcinomas (Klatskin tumors) on the incidence of intra- and extrahepatic cholangiocarcinoma in the United States. J Natl Cancer Inst 2006;98:873-5. [Crossref] [PubMed]
- Tyson GL, Ilyas JA, Duan Z, et al. Secular trends in the incidence of cholangiocarcinoma in the USA and the impact of misclassification. Dig Dis Sci 2014;59:3103-10. [Crossref] [PubMed]
- Khan SA, Emadossadaty S, Ladep NG, et al. Rising trends in cholangiocarcinoma: is the ICD classification system misleading us? J Hepatol 2012;56:848-54. [Crossref] [PubMed]
- Petrick JL, Kelly SP, Altekruse SF, et al. Future of Hepatocellular Carcinoma Incidence in the United States Forecast Through 2030. J Clin Oncol 2016;34:1787-94. [Crossref] [PubMed]
- Patel T. Worldwide trends in mortality from biliary tract malignancies. BMC Cancer 2002;2:10. [Crossref] [PubMed]
- Yao KJ, Jabbour S, Parekh N, et al. Increasing mortality in the United States from cholangiocarcinoma: an analysis of the National Center for Health Statistics Database. BMC Gastroenterol 2016;16:117. [Crossref] [PubMed]
- National Cancer Institute. Surveillance Research Program, Surveillance Systems Branch. Surveillance, Epidemiology, and End Results (SEER) Program Populations (1969-2014). Available online: https://seer.cancer.gov/popdata/
- Vitale A, Moustafa M, Spolverato G, et al. Defining the possible therapeutic benefit of lymphadenectomy among patients undergoing hepatic resection for intrahepatic cholangiocarcinoma. J Surg Oncol 2016;113:685-91. [Crossref] [PubMed]
- Kim Y, Spolverato G, Amini N, et al. Surgical Management of Intrahepatic Cholangiocarcinoma: Defining an Optimal Prognostic Lymph Node Stratification Schema. Ann Surg Oncol 2015;22:2772-8. [Crossref] [PubMed]
- Rutherford MJ, Lambert PC, Thompson JR. Age–period–cohort modeling. Stata J 2010;10:606-27.
- Carstensen B. Age-period-cohort models for the Lexis diagram. Stat Med 2007;26:3018-45. [Crossref] [PubMed]
- McGlynn KA, Tarone RE, El-Serag HB. A comparison of trends in the incidence of hepatocellular carcinoma and intrahepatic cholangiocarcinoma in the United States. Cancer Epidemiol Biomarkers Prev 2006;15:1198-203. [Crossref] [PubMed]
- Dorak MT, Karpuzoglu E. Gender differences in cancer susceptibility: an inadequately addressed issue. Front Genet 2012;3:268. [Crossref] [PubMed]
- Shaib YH, Davila JA, McGlynn K, et al. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol 2004;40:472-7. [Crossref] [PubMed]
- Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis 2004;24:115-25. [Crossref] [PubMed]
- Campbell CA, Canary L, Smith N, et al. State HCV Incidence and Policies Related to HCV Preventive and Treatment Services for Persons Who Inject Drugs - United States, 2015-2016. MMWR Morb Mortal Wkly Rep 2017;66:465-9. [Crossref] [PubMed]
- Centers for Disease Control and Prevention. Surveillance for Viral Hepatitis – United States, 2015. Available online: https://www.cdc.gov/hepatitis/statistics/2015surveillance/commentary.htm
- Reddy SK, Hyder O, Marsh JW, et al. Prevalence of nonalcoholic steatohepatitis among patients with resectable intrahepatic cholangiocarcinoma. J Gastrointest Surg 2013;17:748-55. [Crossref] [PubMed]
- Yu JB, Gross CP, Wilson LD, et al. NCI SEER public-use data: applications and limitations in oncology research. Oncology (Williston Park) 2009;23:288-95. [PubMed]