Targeting gut microbiota in hepatocellular carcinoma: probiotics as a novel therapy
Introduction
Hepatocellular carcinoma (HCC) or primary liver cancer is the fifth most common cancer, and the third most common cause of cancer-related mortality worldwide with over 700,000 deaths each year (1). HCC commonly occurs in chronically damaged liver tissues due to chronic regenerative and inflammatory processes that contribute to the initiation and/or progression of HCC (2). Eighty to ninety percent of all cases of HCC are linked to cirrhosis or fibrosis, and the well-recognized risk factors are infections with hepatitis C virus (HCV), hepatitis B virus (HBV), alcoholic cirrhosis, as well as hemochromatosis (3). Therapy for HCC is complex and can involve surgical resection, liver transplantation, radiofrequency ablation (RFA) and trans-arterial chemoembolization, radioembolization and emerging systemic chemotherapeutic and targeted agents such as sorafenib. However, tumor relapse, metastasis and chemoresistance are common and thus survival rates in patients with advanced HCC are very low. Therefore, HCC is undoubtedly a disease for which there is an urgent need for the development of alternative therapies.
The human intestine offers a protected, warm, and nutrient-rich microenvironment to a complex and dynamic community of around 100 trillion (1014) of microorganisms of diverse taxonomy (2,000 distinct species). These microorganisms are usually referred to as gut microbiota. Moreover, the collective genomes, also known as human microbiome, contain 100–150-fold more genes than the human genome (4), and indeed, it is sometimes referred to as our “forgotten organ”. Commensal bacteria colonize its host immediately after birth and are essential to host development and health. Gut microbiota are essential components to a healthy body, such as assisting digestion, producing vitamins, degrading bile acids, modulating local and general immunity and even treating diseases, such as cancer (5-7). The gut microbiota composition varies significantly among individuals; however, studies showed that there is a core set of gut colonizers shared among all healthy adults, constituting a “core microbiota”. In healthy humans, the dominant bacterial phyla are Firmicutes (30–50%), Bacteroidetes (20–40%) and Actinobacteria (1–10%) (6). Interestingly, gut microbiota composition vary significantly from lower small intestine to distal colon, which are likely affected by both microenvironment such as pH, nutrient availability and intestinal motility (8). There are many factors that can influence the gut microbiota composition, including diet, age, medications, illnesses, stress and lifestyle (9). Microbiome research has greatly advanced our understanding on the complexity and diversity of the gut microbial communities, as well as the interactions between gut microbial communities and their hosts.
Maintenance of a balanced microbiota composition is vital as an ecological barrier to insults from the external environment. A disruption to this micro-ecology, termed intestinal dysbiosis (disequilibrium in microbiota), impairs intestinal homeostasis and leads to overgrowth of certain detrimental bacteria known to induce a variety of diseases, including liver pathology. Indeed, the gut microbiota composition is altered in many liver diseases, thus restructuring the gut microbiota is an emerging target for therapy. Probiotics, as a functional food ingredient, may beneficially influence the gut microbiota and modulate pathogenesis of chronic liver diseases (10), and emerging evidence has indicated that probiotics may also be used as a therapeutic approach for HCC (11). In this review, the interplay of gut microbiota with HCC, and the possible therapeutic implications of probiotics for HCC will be discussed.
Role of gut microbiota in liver diseases: crosstalk between the liver and gut
The gut and the liver are important organs for nutrient absorption and metabolism. Emerging evidence suggest that there is a strong association between liver and gut. The liver receives approximately 75% of its blood supply from the hepatic portal vein (the blood vessel that carries blood from the intestines to the liver). The intestinal blood carries the nutrients from the gut to activate liver functions to augment the effective hepatic processing of nutrients. The liver, in turn, influences intestinal functions by secreting bile into the intestinal lumen (12). When the intestinal barrier is compromised, there will be an increase in intestinal permeability, leading to the translocation of gut-derived bacterial products such as lipopolysaccharides (LPS) via the portal vein (13). Because of the functional link to the intestine, the liver is the first organ barrier against the gut derived bacteria or their metabolites, which are persistently released into the circulation.
Accumulating evidence has suggested that the gut-liver axis plays a pivotal role in the pathogenesis of liver diseases, including hepatitis, non-alcoholic steatohepatitis (NASH), liver cirrhosis, and HCC (13-17). Liver diseases have long been associated with qualitative (dysbiosis) and quantitative (overgrowth) changes in gut microbiota (18). Alterations of gut microbiota were first identified in patients with chronic liver disease almost a decade ago. Derangement of the gut flora, especially small intestinal bacterial overgrowth (SIBO), happens in a high proportion (20–75%) of patients with chronic liver disease (14). Bacterial overgrowth correlates with an increase in the luminal amount of LPS, a pathogen-associated molecular pattern (PAMP) of the gut-liver axis. In the presence of an intestinal barrier dysfunction, the luminal bacterial load determines the amount of LPS that translocate to the systemic circulation, and to mesenteric lymph nodes or other extra-intestinal organs, and hence the degree of liver damage (12,19). Elevated levels of LPS in the portal and/or systemic circulation were noted in several types of chronic liver disease (14). Endotoxemia appears to be responsible for initiation of the liver damage, probably by interacting with specific recognition receptors, such as the toll like receptors (TLR) (20), although TLR independent mechanisms by which gut microbiota produces liver injury has been described (21). Alteration of gut microbiota can increase intestinal permeability to LPS that, in turn, activates the kupffer cells, the liver macrophages to release pro-inflammatory cytokines, such as tumour necrosis factor alpha (TNFα) and reactive oxygen intermediates, which will lead to pathogenesis of various acute and chronic liver diseases (12). Currently, it is not entirely clear how alterations in the gut microbiota contribute to the pathogenesis of chronic liver diseases and HCC in humans.
Role of gut microbiota in HCC development
There is an increasing incidence in HCC and it is often associated with a very high fatality rate. Cirrhosis due to HBV or HCV is the major risk factor for HCC. However, as obesity and NAFLD has become more prevalent in developed countries, the incidence of HCC is also increasing in these regions (22). In viral hepatitis, it is thought that chronic inflammation of the liver is linked to hepatocarcinogenesis; however, the exact mechanism for the development of HCC in NAFLD patients has yet been elucidated.
To date, several experimental data from animal models as well as human studies suggest the role of gut microbiota in the development of HCC. Intestinal dysbiosis was found in patients with liver cirrhosis and HCC and in mice after diethylnitrosamine (DEN) administration. An imbalance in gut microbiota composition included a significant suppression of Lactobacillus species, Bifidobacterium species and Enterococcus species and a significant growth of E. coli and Atopobium cluster (23). Gut sterilization decreased HCC development when mice were treated with DEN and hepatotoxin carbon tetrachloride (CCl4), a model that mimics the microenvironment of the majority of human HCC. The involvement of intestinal microbiota was also confirmed by reduced hepatocarcinogenesis in germ-free mice in comparison with specific pathogen-free mice (24). In addition, a study by Fox et al. has suggested the relationship between intestinal colonisation by Helicobacter hepaticus and induction of HCC (25). Huang et al. reported the presence of Helicobacter ssp. 16S rDNA in the liver of HCC patients, but absent from that of control (26). However, in another study by Krüttgen et al., the authors failed to detect Helicobacter hepaticus in the stool samples of HCC patients with HBV or HCV infection (27). Hence, additional studies on a possible link between gut microbiota and HCC in humans are necessary.
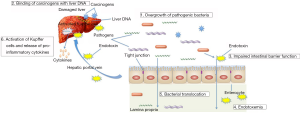
As discussed, alterations in gut microbiota and endotoxemia are critical components in promoting the pathogenesis of chronic liver diseases and HCC. Here, the mechanisms through which the gut-liver axis promotes HCC pathogenesis will be discussed and outlined in Figure 1.
HCC promotion via leaky gut, endotoxemia and TLR
High circulating levels of LPS were shown in both animal models of carcinogen-induced hepatocarcinogenesis and HCC patients (23,28). This accumulation is likely attributed to increases in the intestinal permeability and bacterial translocation, together with deficient clarification of the hepatic reticuloendothelial system (29). Accumulation of LPS contributes to the pathogenesis of HCC by provoking pro-inflammatory actions in the liver (28).
TLRs are pattern recognition receptors that recognize endotoxins and signals through the MyD88 (myeloid differentiation primary-response protein 88)-dependent and MyD88-independent pathways, which activate innate immunity (30). The liver is well equipped to respond to endotoxins, with Kupffer cells being more responsive to endotoxins than hepatocytes (28). Recent studies have suggested the involvement of TLR4 in hepatocarcinogenesis. Overexpression of TLR4 was shown in tumor tissues of HCC patients (31,32). A recent research by Liu et al. demonstrated a strong correlation of TLR4 expression levels with microvascular invasion, early recurrence and poor survivals in HCC patients (33). Another study has also reported that LPS-induced TLR4 signaling promoted cancer cell survival and proliferation in HCC (34). Activation of TLR4 signaling pathways results in the production of pro-inflammatory immune mediators, which may contribute to the development and progression of hepatocarcinogenesis (35). Significant decreases in the incidence, size, and number of chemical-induced liver cancer were noted in mice deficient in TLR4 and MyD88, but not TLR2, implying there is a strong association of TLR signaling to hepatocarcinogenesis (36). Dapito et al. also clearly demonstrated that LPS-induced TLR4 activation is required for hepatocarcinogenesis in the chronically injured liver, and TLR4 were not necessary for HCC initiation but for HCC promotion involving hepatocyte proliferation and evasion of apoptosis (24).
HCC promotion via dysbiosis and bacterial metabolite production
Recent evidence suggests that gut microbiota are involved in HCC. Intestinal dysbiosis was observed in rats with chronic DEN treatment. This was often associated with an increase in pathogenic bacteria, together with decreased numbers of beneficial ones (23). Administration of penicillin or dextran sulfate sodium (DSS) was shown to disrupt the balance of gut microbiota and cause mucosa damage, led to the development of endotoxemia, systemic inflammation and tumor formation (23).
It is also evident that intestinal dysbiosis affects the development of liver disease and HCC through bacterial metabolite production. In a study by Yoshimoto et al., dietary or genetic obesity alters gut microbiota and thus rises the deoxycholic acid (DCA) levels, a gut bacterial metabolite that cause DNA damage. DCA provokes the production of various inflammatory and tumour-promoting factors in the liver, thereby promoting HCC development in mice after exposure to chemical carcinogen. In the same model, blocking DCA production or reducing gut microbiota efficiently prevents HCC development in obese mice (37). On the other hand, it has been shown that propionate, a metabolite from gut microbiota, could inhibit cancer cell proliferation in the liver (38).
HCC promotion via immunomodulation
HCC is usually derived from inflamed fibrotic and/or cirrhotic liver with intensive immune cell infiltration. Thus, the immune status may have a large impact on the biologic behaviour of HCC. It has been reported that tumor-associated macrophages (39), neutrophils (40), natural killer (NK) cells (41,42), regulatory T cells (43,44) and interleukin 6 (IL6) (45) were associated with poor prognosis of HCC patients.
T helper 17 (Th17) cells is a novel subset of T helper cells that produces pro-inflammatory and pro-angiogenic mediators such as IL17A and IL22. Elevated level of Th17 cells have been detected in tumors and blood of HCC patients (46,47) and the levels of Th17 cells were positively correlated with poor survival. IL17A secreted by Th17 cells may play pro-tumor roles by promoting tumor angiogenesis by secreting angiogenic mediators and cytokines (48). The abundance of Th17 cells in the healthy liver is low, majority of these Th17 cells are produced in the gut through interaction with gut microbiota (49). Consequently, it is postulated that it is the IL17 produced from the intestine that may involve in the development of HCC. Taken together, the above-described findings show that the immune status may vary in different tumor microenvironments and have different impacts on disease progression, and gut microbiota can exert a profound impact in the development of HCC via immunomodulation. Owing to a lack of pharmaco preventive strategies and limited chemotherapeutic options for treatment of liver cancer, the therapeutic modulation of the gut microflora by antibiotics, prebiotics or probiotics might represent novel approaches to prevent the progression from chronic hepatitis to liver cirrhosis and HCC.
Probiotics as a novel approach for preventing/ treating HCC
Probiotics are defined as “live microorganisms which when administered in adequate amounts confer a health benefit for the host” by United Nations and WHO (50). In another words, a bacterium (or any microbe) needs to be isolated, purified, characterized, and proved to be beneficial to health when it is administered before it can be designated as a probiotic. Successful probiotics strains need to be able to survive passage through the upper gastrointestinal tract, multiply, colonize and function in the gut. They are mostly of human origin (51,52). Strains of lactic acid bacteria, especially Lactobacillus and Bifidobacterium species are frequently employed as probiotics in fermented dairy products (53).
The popularity of probiotics has expanded exponentially recently, due to their health promoting effects and their abilities to prevent or treat different diseases (54,55). The gut microbiota composition is altered in many liver diseases, restructuring the gut microbiota represents a new potential therapeutic target. Beneficial effects of probiotics have been reported not only in gastrointestinal diseases but also liver diseases. There are several reviews summarising the benefits of probiotics on liver diseases (20,53,56-61). The exact mechanisms of beneficial effects of probiotics on the gut-liver axis are yet to be fully revealed, probiotics may exert their therapeutic effects through (I) modulation of gut microbiota composition and antimicrobial factor production; (II) improvement of gut barrier function; and (III) modulation of local and systemic immunity (53). The beneficial effects of probiotics are often species-specific or even strain-specific (62). Therefore, the choice of strain or combination of strains is crucial for therapeutic success.
The application of probiotics to modulate tumor growth has been demonstrated. Efficacy of probiotics on cancer development is mostly associated with colon cancer. Yet, as gut microbiota may affect the immune system systemically (63,64), the effects of probiotics on other cancer types have also drawn researchers’ attention.
Regarding liver cancer, there are few studies regarding the use of probiotic supplement as a dietary approach to reduce the risk of HCC induced by aflatoxins. For example, in a clinical study by El-Nezami et al., dietary supplementation using probiotics such as viable Lactobacillus rhamnosus LC705 and Propionibacterium freudenreichii subsp. Shermani) could effectively reduce the excretion of aflatoxin-DNA adduct (AFB1-N7-guanine) in urine (65). In a rat study investigating the chemopreventive effect of probiotic-fermented milk and chlorophyllin on AFB1-induced HCC, the probiotics treatment reduced the tumor incidence, as well as decreased c-myc, bcl-2, cyclin D1 and rasp-21 level, which suggests the protective capacity of probiotics against AFB1-induced hepatocarcinogenesis (66). In another rat study, administration of VSL#3 (containing (four Lactobacilli, three Bifidobacteria, and one Streptococcus thermophilus subsp Salivarius) mitigated DEN-induced hepatocarcinogenesis by restoring gut homeostasis and ameliorating intestinal and hepatic inflammation, and thus inhibiting the progression of cirrhosis to HCC (23).
Recently, Li et al. reported that probiotics inhibit HCC progression in mice (11). Feeding the probiotics mixture Prohep (comprising Lactobacillus rhamnosus GG, Escherichia coli Nissle 1917 and heat-inactivated VSL#3) to tumor-injected mice could shift the gut microbiota composition and reduce size of liver tumors. In addition to the reduction of tumor size, angiogenic factors were down-regulated by probiotics administration. Concerning the mechanistic pathways, the level of Th17 cells in gut and recruitment of Th17 to tumor site were lower in probiotics-treated mice. The anti-cancer effect of probiotics was also associated with the short chain fatty acids (SCFA) production, which could be reflected by the enrichment of SCFA-related pathway that was found in probiotics-treated mice.
Mechanisms of action of probiotics against HCC
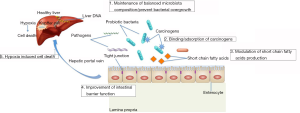
There are several mechanisms responsible for the anti-cancer effects of probiotics. Other than the direct impact of probiotics on modulating gut microbiota, probiotics can exert its anti-cancer actions through immune modulation, decreased bacterial translocation, improved intestinal barrier function, anti-inflammatory and anti-pathogenic activities, as well as reducing tumor formation and metastasis [for review, see (67)]. In this section, the mechanisms of action of probiotics against HCC will be summarized and outlined in Figure 2.
Binding/adsorption of carcinogens
There are few studies showing that the ability of probiotics to bind and immobilise toxic compounds with the gut lumen. As such, the deleterious effects of dietary toxic substances could be reduced and thus lead to an improvement of gut and liver health. In the past 20 years, more than 250 strains of lactic acid bacteria isolated from either dairy products or healthy human subjects were screened in our laboratories and several bacterial strains that were capable of binding a range of mycotoxins were identified (68-71). These bacterial strains were subsequently demonstrated to bind mycotoxins ex vivo in ligated duodenal loops of 1 week old chicks and reduced its uptake into intestinal tissue by 74% (72). The damage to intestinal epithelia following exposure to mycotoxins could be attenuated in the presence of probiotics bacteria. Incubating cytochrome P450 3A4 (CYP3A4) induced Caco-2 monolayer with aflatoxin B1 (AFB1) significantly decreased the trans-epithelial electrical resistance (TEER) (73). In the presence of probiotic bacteria, this aflatoxin-induced decrease in TEER was attenuated, indicating that probiotic bacteria could decrease aflatoxin-induced cytotoxicity. The above in vitro results led to a series of in vivo studies which investigated of the potential capacity of selected strains to bind AFB1 as a mycotoxin model and to examine if the binding strength was sufficient to decrease AFB1 bioavailability. Results from the animal studies have shown that both AFB1 toxicity and bioavailability is reduced by probiotic supplementation (74). Dietary supplementation of probiotic bacteria to Chinese subjects exposed to AFB1 via diet was also shown to effectively reduce the urinary excretion of aflatoxin-DNA adduct (AFB1-N7-guanine), a well-validated biomarker for liver cancer risk (75). These studies suggest that probiotic supplementation reduced the bioavailability of carcinogen AFB1, and thus reduce HCC risk.
Improvement of intestinal barrier function
As previously mentioned, the liver is under constant exposure to gut-derived bacterial products, thus maintaining an effective gut barrier is crucial which restricts the amount of bacterial components reaching the liver. In advanced liver diseases, the intestinal barrier function is disrupted; there will be an increase in intestinal permeability to gut-derived LPS. Accumulation of LPS contributes to the HCC pathogenesis by provoking pro-inflammatory responses in the liver (28). Interestingly, a study by Zhang et al. demonstrated that probiotics could reshape gut microbiota composition, suppressing the outgrowth of Gram-negative bacteria and enhancing intestinal barrier that prevents translocation of endotoxins. As a result, tumorigenic inflammation in the liver was reduced (23).
Modulation of SCFA production
The gut microbiota is exclusively responsible for several important metabolic functions, mainly by fermentation of dietary non-digestible carbohydrates. In the human gut, the end products of carbohydrate fermentation include a range of organic acids, including SCFA, mainly acetate, propionate and butyrate (76). Butyrate is the primary energy source for colonocytes. Propionate is almost totally taken up by the liver from the bloodstream, whereas acetate enters the peripheral circulation and is largely metabolized by peripheral tissues (77). Certain strains of probiotics such as bifidobacteria and lactobacilli can shift the gut microbiota composition and thus the production of SCFA (78). The production of SCFA by these bacteria may reduce the risk of developing cancer, including HCC. Li et al. revealed that the anti-cancer effect of probiotics was associated with SCFA production. Certain genera of bacteria, including Butyricimonas and Prevotella were enriched, which can generate anti-inflammatory fatty acids such as butyrate and propionate. Pathway analyses have shown that probiotics tend to strengthen metabolism related to SCFA synthesis, tricarboxylic acid (TCA) cycle, carboxylate degradation, etc. (11), which may offer protection against the development of HCC.
Regulation of Th17 response
As discussed, Th17 cells have recently emerged as an important T helper cell subset that are defined by their production of pro-inflammatory and pro-angiogenic mediators such as IL17A, IL22. Increased Th17 densities have been detected in HCC tumors, where their levels were associated with poor disease outcome. IL17-producing cells are accumulated in HCC, where they may promote tumor progression by fostering angiogenesis (23,79). Li et al. clearly demonstrated that a probiotics mixture that inhibited HCC progression reduced level of pro-inflammatory cytokines, IL-17, in mice. Probiotics caused shifts in the gut microbiota composition toward specific beneficial bacteria, for example, Prevotella and Oscillibacter. These bacteria are known to produce anti-inflammatory metabolites, which subsequently decreased the Th17 polarization and favored the differentiation of anti-inflammatory Treg/Type 1 regulatory T (Tr1) cells in the gut. Apart from regulating T cell polarization, probiotics worked by controlling the growth of segmented filamentous bacteria (SFB), the major Th17-inducing bacteria. Indeed, administration of probiotics dramatically decreased the SFB. Consequently, the pro-inflammatory cytokine IL17 production was reduced (11). As IL17A produced from Th17 favour angiogenesis (80), reduction of Th17 and IL17 level may contribute to inhibition of cancer progression.
Hypoxia-induced cell death
Hypoxia is a defining feature of solid tumors, which the tumor cells are deficient in oxygen. Tumor hypoxia can lead to therapeutic problems because it often makes solid tumors resistant to conventional cancer treatments. Tumor hypoxia appears to be correlated with tumor propagation and malignant progression (81). Evidence suggests that hypoxia inhibits tumor cell differentiation and facilitates stem cell maintenance (82). In the study by Li et al., mice after probiotic administration showed increased hypoxia of liver tumor cells. The increase in tumor hypoxia was due to the weakened angiogenesis and thus limiting tumor growth. In addition, an elevated expression of the hypoxia-inducible factor 1 (HIF-1) was also noted in probiotics-treated mice, which could also account for the induction of glucose transporter 1 (GLUT-1), a molecular marker to indicate the degree of hypoxia (11). This study provides a mechanistic insight for utilization of probiotics for the treatment of HCC, by enhancing hypoxia-induced cell death.
Conclusions and future perspective
In conclusion, the gut-liver axis plays an important role in the pathogenesis of liver diseases, including HCC. Growing evidence has supported the role of gut microbiota in the development of HCC. Thus, manipulation of the gut microbiota may represent a novel way to treat or prevent HCC. Probiotics may represent innovative, safe and low cost strategies to prevent or treat HCC. However, more laboratory-based mechanistic studies as well as extensive human clinical trials in evaluation of gut microbiota and appropriately selected useful bacterial strains are necessary to gain the acceptance of the broader medical community and to investigate the possibility of using probiotics as an alternative therapeutic method for cancer. Moreover, it is worth noting that molecular pathological epidemiology (MPE) is an emerging field that examines the relationship between different endogenous (including microbiota), environmental, and lifestyle factors and tumor molecular changes (83). With the advancements of high-throughput sequencing technologies, it allows integration of various disciplines into MPE (e.g., microbial MPE), which can provide important etiologic and pathogenic insights of any diseases, including HCC, and potentially contributing to precision medicine (84).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- White DL, Kanwal F, Jiao L, et al. Epidemiology of hepatocellular carcinoma. In: Carr BI, editor. Hepatocellular carcinoma: Diagnosis and treatment. Cham: Springer International Publishing 2016;3-24.
- Castven D, Fischer M, Becker D, et al. Adverse genomic alterations and stemness features are induced by field cancerization in the microenvironment of hepatocellular carcinomas. Oncotarget 2017;8:48688-700. [Crossref] [PubMed]
- Adam R, Azoulay D, Castaing D, et al. Liver resection as a bridge to transplantation for hepatocellular carcinoma on cirrhosis: a reasonable strategy? Ann Surg 2003;238:508. [PubMed]
- Qin J, Li R, Raes J, et al. A human gut microbial gene catalog established by metagenomic sequencing. Nature 2010;464:59-65. [Crossref] [PubMed]
- Pennisi E. Cancer therapies use a little help from microbial friends. Science 2013;342:921. [Crossref] [PubMed]
- Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature 2012;489:242-9. [Crossref] [PubMed]
- Abt MC, Artis D. The intestinal microbiota in health and disease: the influence of microbial products on immune cell homeostasis. Curr Opin Gastroenterol 2009;25:496. [Crossref] [PubMed]
- Son G, Kremer M, Hines IN. Contribution of gut bacteria to liver pathobiology. Gastroenterol Res Pract 2010.2010. [PubMed]
- Conlon MA, Bird AR. The impact of diet and lifestyle on gut microbiota and human health. Nutrients 2014;7:17-44. [Crossref] [PubMed]
- Loguercio C, De Simone T, Federico A, et al. Gut-liver axis: a new point of attack to treat chronic liver damage? Am J Gastroenterol 2002;97:2144. [Crossref] [PubMed]
- Li J, Sung CYJ, Lee N, et al. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. PNAS 2016;113:E1306-15. [Crossref] [PubMed]
- Zeuzem S. Gut-liver axis. Int J Colorectal Dis 2000;15:59-82. [Crossref] [PubMed]
- Seki E, Schnabl B. Role of innate immunity and the microbiota in liver fibrosis: crosstalk between the liver and gut. J Physiol 2012;590:447-58. [Crossref] [PubMed]
- Compare D, Coccoli P, Rocco A, et al. Gut--liver axis: the impact of gut microbiota on non alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis 2012;22:471-6. [Crossref] [PubMed]
- Chassaing B, Etienne-Mesmin L, Gewirtz AT. Microbiota-liver axis in hepatic disease. Hepatology 2014;59:328-39. [Crossref] [PubMed]
- Szabo G. Gut–liver axis in alcoholic liver disease. Gastroenterology 2015;148:30-6. [Crossref] [PubMed]
- Szabo G, Bala S. Alcoholic liver disease and the gut-liver axis. World J Gastroenterol 2010;16:1321-9. [Crossref] [PubMed]
- Schnabl B, Brenner DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology 2014;146:1513-24. [Crossref] [PubMed]
- Llorente C, Schnabl B. The gut microbiota and liver disease. Cell Mol Gastroenterol Hepatol 2015;1:275-84. [Crossref] [PubMed]
- Cesaro C, Tiso A, Del Prete A, et al. Gut microbiota and probiotics in chronic liver diseases. Dig Liver Dis 2011;43:431-8. [Crossref] [PubMed]
- Bilzer M, Roggel F, Gerbes AL. Role of Kupffer cells in host defense and liver disease. Liver Int 2006;26:1175-86. [Crossref] [PubMed]
- Mittal S, El-Serag HB. Epidemiology of HCC: Consider the population. J Clin Gastroenterol 2013;47:S2-6. [Crossref] [PubMed]
- Zhang HL, Yu LX, Yang W, et al. Profound impact of gut homeostasis on chemically-induced pro-tumorigenic inflammation and hepatocarcinogenesis in rats. J Hepatol 2012;57:803-12. [Crossref] [PubMed]
- Dapito DH, Mencin A, Gwak GY, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell 2012;21:504-16. [Crossref] [PubMed]
- Fox JG, Feng Y, Theve EJ, et al. Gut microbes define liver cancer risk in mice exposed to chemical and viral transgenic hepatocarcinogens. Gut 2010;59:88-97. [Crossref] [PubMed]
- Huang Y, Fan XG, Wang ZM, et al. Identification of helicobacter species in human liver samples from patients with primary hepatocellular carcinoma. J Clin Pathol 2004;57:1273-7. [Crossref] [PubMed]
- Krüttgen A, Horz HP, Weber-Heynemann J, et al. Study on the association of helicobacter species with viral hepatitis-induced hepatocellular carcinoma. Gut Microbes 2012;3:228-33. [Crossref] [PubMed]
- Yu LX, Yan HX, Liu Q, et al. Endotoxin accumulation prevents carcinogen-induced apoptosis and promotes liver tumorigenesis in rodents. Hepatology 2010;52:1322-33. [Crossref] [PubMed]
- Almeida J, Galhenage S, Yu J, et al. Gut flora and bacterial translocation in chronic liver disease. World J Gastroenterol 2006;12:1493-502. [Crossref] [PubMed]
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol 2004;4:499-511. [Crossref] [PubMed]
- Yang J, Zhang JX, Wang H, et al. Hepatocellular carcinoma and macrophage interaction induced tumor immunosuppression via Treg requires TLR4 signaling. World J Gastroenterol 2012;18:2938-47. [Crossref] [PubMed]
- Eiró N, Altadill A, Juárez LM, et al. Toll-like receptors 3, 4 and 9 in hepatocellular carcinoma: Relationship with clinicopathological characteristics and prognosis. Hepatol Res 2014;44:769-78. [Crossref] [PubMed]
- Liu WT, Jing YY, Yu GF, et al. Toll like receptor 4 facilitates invasion and migration as a cancer stem cell marker in hepatocellular carcinoma. Cancer Lett 2015;358:136-43. [Crossref] [PubMed]
- Wang L, Zhu R, Huang Z, et al. Lipopolysaccharide-induced toll-like receptor 4 signaling in cancer cells promotes cell survival and proliferation in hepatocellular carcinoma. Dig Dis Sci 2013;58:2223-36. [Crossref] [PubMed]
- Yang J, Li M, Zheng QC. Emerging role of Toll-like receptor 4 in hepatocellular carcinoma. J Hepatocell Carcinoma 2015;2:11-7. [PubMed]
- Naugler WE, Sakurai T, Kim S, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science 2007;317:121-4. [Crossref] [PubMed]
- Yoshimoto S, Loo TM, Atarashi K, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 2013;499:97-101. [Crossref] [PubMed]
- Bindels LB, Porporato P, Dewulf EM, et al. Gut microbiota-derived propionate reduces cancer cell proliferation in the liver. Br J Cancer 2012;107:1337-44. [Crossref] [PubMed]
- Zhu XD, Zhang JB, Zhuang PY, et al. High expression of macrophage colony-stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. J Clin Oncol 2008;26:2707-16. [Crossref] [PubMed]
- Li YW, Qiu SJ, Fan J, et al. Intratumoral neutrophils: A poor prognostic factor for hepatocellular carcinoma following resection. J Hepatol 2011;54:497-505. [Crossref] [PubMed]
- Chew V, Chen J, Lee D, et al. Chemokine-driven lymphocyte infiltration: an early intratumoural event determining long-term survival in resectable hepatocellular carcinoma. Gut 2012;61:427-38. [Crossref] [PubMed]
- Taketomi A, Shimada M, Shirabe K, et al. Natural killer cell activity in patients with hepatocellular carcinoma. Cancer 1998;83:58-63. [Crossref] [PubMed]
- Gao Q, Qiu SJ, Fan J, et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol 2007;25:2586-93. [Crossref] [PubMed]
- Fu J, Xu D, Liu Z, et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology 2007;132:2328-39. [Crossref] [PubMed]
- Pang XH, Zhang JP, Zhang YJ, et al. Preoperative levels of serum interleukin-6 in patients with hepatocellular carcinoma. Hepatogastroenterology 2011;58:1687-93. [PubMed]
- Zhang JP, Yan J, Xu J, et al. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol 2009;50:980-9. [Crossref] [PubMed]
- Liao R, Sun J, Wu H, et al. High expression of IL-17 and IL-17RE associate with poor prognosis of hepatocellular carcinoma. J Exp Clin Cancer Res 2013;32:3. [Crossref] [PubMed]
- Ye J, Livergood RS, Peng G. The role and regulation of human Th17 cells in tumor immunity. Am J Pathol 2013;182:10-20. [Crossref] [PubMed]
- Ivanov II, Atarashi K, Manel N, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 2009;139:485-98. [Crossref] [PubMed]
- Guidelines for the Evaluation of Probiotics in Food. Available online: http://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf
- Saarela M, Mogensen G, Fonden R, et al. Probiotic bacteria: safety, functional and technological properties. J Biotechnol 2000;84:197-215. [Crossref] [PubMed]
- Reuter G. The Lactobacillus and Bifidobacterium microflora of the human intestine: composition and succession. Curr Issues Intest Microbiol 2001;2:43-53. [PubMed]
- Kirpich IA, McClain CJ. Probiotics in the treatment of the liver diseases. J Am Coll Nutr 2012;31:14-23. [Crossref] [PubMed]
- Sanders ME, Guarner F, Guerrant R, et al. An update on the use and investigation of probiotics in health and disease. Gut 2013;62:787-96. [Crossref] [PubMed]
- de Moreno MA. The administration of probiotics and fermented products containing lactic acid bacteria exert beneficial effects against intestinal and non-intestinal cancers. J Food Nutr Disor 2014:S1-005.
- Nitin J, Mithun S, Rao P, et al. Liver diseases: The role of gut microbiota and probiotics. J Prob Health 2016;4:2. [Crossref]
- Elzouki AN. Probiotics and liver disease: Where are we now and where are we going? J Clin Gastroenterol 2016;50:S188-90. [Crossref] [PubMed]
- Gratz SW, Mykkanen H, El-Nezami HS. Probiotics and gut health: a special focus on liver diseases. World J Gastroenterol 2010;16:403-10. [Crossref] [PubMed]
- Imani Fooladi AA, Mahmoodzadeh Hosseini H, Nourani MR, et al. Probiotic as a novel treatment strategy against liver disease. Hepat Mon 2013;13:e7521. [Crossref] [PubMed]
- Sheth AA, Garcia-Tsao G. Probiotics and liver disease. J Clin Gastroenterol 2008;42:S80-4. [Crossref] [PubMed]
- Sharma V, Garg S, Aggarwal S. Probiotics and liver disease. Perm J 2013;17:62-7. [Crossref] [PubMed]
- Hill C, Guarner F, Reid G, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 2014;11:506-14. [Crossref] [PubMed]
- Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science 2012;336:1268-73. [Crossref] [PubMed]
- Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol 2016;16:341. [Crossref] [PubMed]
- de LeBlanc AdM. Matar C, Perdigón G. The application of probiotics in cancer. Br J Nutr 2007;98:S105-10. [PubMed]
- Kumar M, Verma V, Nagpal R, et al. Effect of probiotic fermented milk and chlorophyllin on gene expressions and genotoxicity during AFB1-induced hepatocellular carcinoma. Gene 2011;490:54-9. [Crossref] [PubMed]
- Yu AQ, Li L. The potential role of probiotics in cancer prevention and treatment. Nutr Cancer 2016;68:535-44. [Crossref] [PubMed]
- El-Nezami H, Kankaanpaa P, Salminen S, et al. Physicochemical alterations enhance the ability of dairy strains of lactic acid bacteria to remove aflatoxin from contaminated media. J Food Prot 1998;61:466-8. [Crossref] [PubMed]
- El-Nezami H, Kankaanpaa P, Salminen S, et al. Ability of dairy strains of lactic acid bacteria to bind a common food carcinogen, aflatoxin B1. Food Chem Toxicol 1998;36:321-6. [Crossref] [PubMed]
- El-Nezami H, Polychronaki N, Salminen S, et al. Binding rather than metabolism may explain the interaction of two food-grade Lactobacillus strains with zearalenone and its derivative alpha-zearalenol. Appl Environ Microbiol 2002;68:3545-9. [Crossref] [PubMed]
- El-Nezami HS, Chrevatidis A, Auriola S, et al. Removal of common Fusarium toxins in vitro by strains of Lactobacillus and Propionibacterium. Food Addit Contam 2002;19:680-6. [Crossref] [PubMed]
- El-Nezami H, Mykkänen H, Kankaanpää P, et al. Ability of Lactobacillus and Propionibacterium strains to remove aflatoxin B, from the chicken duodenum. J Food Prot 2000;63:549-52. [Crossref] [PubMed]
- Gratz S, Wu QK, El-Nezami H, et al. Lactobacillus rhamnosus strain GG reduces aflatoxin B1 transport, metabolism, and toxicity in Caco-2 cells. Appl Environ Microbiol 2007;73:3958-64. [Crossref] [PubMed]
- Gratz S, Täubel M, Juvonen RO, et al. Lactobacillus rhamnosus strain GG modulates intestinal absorption, fecal excretion, and toxicity of aflatoxin B1 in rats. Appl Environ Microbiol 2006;72:7398-400. [Crossref] [PubMed]
- El-Nezami HS, Polychronaki NN, Ma J, et al. Probiotic supplementation reduces a biomarker for increased risk of liver cancer in young men from Southern China. Am J Clin Nutr 2006;83:1199-203. [PubMed]
- Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology 2009;136:65-80. [Crossref] [PubMed]
- Wong JM, De Souza R, Kendall CW, et al. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol 2006;40:235-43. [Crossref] [PubMed]
- LeBlanc JG, Chain F, Martín R, et al. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb Cell Fact 2017;16:79. [Crossref] [PubMed]
- Numasaki M, Fukushi J, Ono M, et al. Interleukin-17 promotes angiogenesis and tumor growth. Blood 2003;101:2620-7. [Crossref] [PubMed]
- Murugaiyan G, Saha B. Protumor vs antitumor functions of IL-17. J Immunol 2009;183:4169-75. [Crossref] [PubMed]
- Höckel M, Vaupel P. Tumor hypoxia: Definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst 2001;93:266-76. [Crossref] [PubMed]
- Kim Y, Lin Q, Glazer PM, et al. Hypoxic tumor microenvironment and cancer cell differentiation. Curr Mol Med 2009;9:425-34. [Crossref] [PubMed]
- Gao C. Molecular pathological epidemiology in diabetes mellitus and risk of hepatocellular carcinoma. World J Hepatol 2016;8:1119-27. [Crossref] [PubMed]
- Ogino S, Nishihara R, VanderWeele TJ, et al. The role of molecular pathological epidemiology in the study of neoplastic and non-neoplastic diseases in the era of precision medicine. Epidemiology 2016;27:602-11. [Crossref] [PubMed]