Synchronous colorectal liver metastases: a national survey of surgeon opinions on simultaneous resection and multidisciplinary cooperation
Introduction
Colorectal cancer is the third most common cancer and the third leading cause of cancer mortality (1,2). Hepatic resection is considered the gold standard for curative intent in patients who present with synchronous disease. Given that the liver is the most common site of distant metastases for the 20% of patients who arrive with synchronous disease, and that liver metastases develop in 50% of patients overall at some point in their care/surveillance (3,4), identifying optimal sequencing and synergies between resection of the colorectal primary and hepatic metastases is essential.
Simultaneous resection of synchronous colorectal liver metastases (sCRLM) remains somewhat controversial. Although it is now evident that the rate of major morbidity and mortality after simultaneous resections is related to the individual threat of both the hepatic and colorectal resections, it is also clear that the magnitude of the hepatic resection drives the majority of this risk (5,6). More specifically, morbidity and mortality are observed to be lower with a minor hepatectomy combined with either a low or high risk colorectal resection (5). It must also be stated however, that simultaneous and staged resections carry an overall comparable morbidity, mortality and long-term oncologic outcome when patients are appropriately and cautiously selected (6).
One of the dominant reasons to consider a simultaneous resection at the time of patient presentation remains the benefit of reducing the total treatment time frame (7). More specifically, the patient’s operative voyage can be shortened by at least one, and sometimes two additional surgical interventions. Although the clinical and psychological benefits of a shorter treatment paradigm have not been well studied in this setting, the potential benefit is striking. As a result, the goal of this study was to better understand a variety of surgeons’ points of view on both the optimal and realistic delivery of simultaneous resections.
Methods
Ethics approval for this study was obtained from the University of Calgary institutional review board. A qualitative survey outlining both general philosophies and specific case scenarios was constructed by a multidisciplinary team of clinicians (colorectal, hepatic, thoracic, and general surgeons). Discussion, review and revision were iterative in nature until the entire panel agreed with the tool. The voluntary survey was then distributed to members of the surgical community across the country with the goal of understanding the viewpoints of colorectal (CRS) compared to other surgeons (general surgeons and non-hepatobiliary surgical oncologists) with regard to the optimal and realistic management of sCRLM. The survey included questions regarding surgeons’ referral practice, operative management, opinions on outcomes, and assessment of patient suitability for various treatments. Patient symptoms associated with the colorectal primary lesion included ongoing hemorrhage, obstructions, near obstruction, and persistent pain. The survey was distributed to the general membership of the Canadian Association of General Surgeons (CAGS) and Canadian Society for Colorectal Surgery (CSCRS) via a monthly newsletter invitation. The surgical subspecialty categorization for each survey respondent was self-reported. The anonymous online survey was available for completion between March 01, 2015 and June 01, 2015. Surgeons were also asked to supply demographic data to allow stratification into groups according to surgical subspecialty, time in practice, experience, size of regional catchment, size of hospital, and province of practice. Respondents were also required to engage in ‘regular’ general surgical call shifts/coverage and not engage in hepatic surgery on a non-emergent basis.
All responses were analyzed as pooled data. Respondents answering less than four questions were excluded. The chi-squared statistic was used to determine statistically significant differences between groups. All statistical testing was performed using Stata/IC version 12.0 (Stata Corp., College Station, TX, USA).
Results
A total of 58 surgeons responded to the survey invitation. Six of these were excluded because less than four responses were completed. Of the remaining 52, 28 (53.8%) identified themselves as colorectal surgeons (CRS). Geographical distribution was: British Columbia—9 (17.3%), Alberta—6 (11.5%), Manitoba—2 (3.8%), Ontario—18 (34.6%), Quebec—8 (15.4%), Newfoundland—1 (1.9%), Nova Scotia—2 (3.8%), New Brunswick—3 (5.8%), and undisclosed—3 (5.8%).
Referral practice
Amongst all respondents, 94.2% had access to hepatobiliary (HPB) surgeons at their own institution (53.8%), geographical area (34.6%), or via telehealth (3.8%). All respondents had access to medical oncologists at their institution (98.1%) or within their geographic area (1.9%).
The majority of those surveyed (94.3%) saw a role for simultaneous resection of sCRLM (55.8% having first-hand experience with these cases). In cases of known hepatic metastases, 59.6% of surgeons stated they discussed more than 75% of cases with HPB surgeons prior to resection of the colorectal primary. In the specific case of an asymptomatic/minimally symptomatic primary with sCRLM, most surgeons consulted medical oncology first (52.9%), while the remainder consulted HPB (37.3%) or resected the primary tumor (9.8%) first. If the primary tumor was symptomatic, 78.4% resected the primary first, while 13.7% consulted HPB first, and the remainder consulted medical oncology (7.8%).
Factors restricting referral to an HPB surgeon were: bilobar or extensive hepatic metastases (55.6%), portal lymphadenopathy (32.7%), extensive patient comorbidities (44.2%), age of the patient (13.5%) and limitations in the accessibility of an HPB surgeon (11.5%).
In a case scenario describing an asymptomatic colorectal primary with sCRLM: 67.4% of respondents overall would refer the patient to an HPB surgeon and medical oncologist for discussion; 13.0% would refer to an HPB surgeon, followed by resection of the primary and subsequent hepatic metastectomy as indicated; 10.9% would resect the primary tumor, followed by referral to an HPB surgeon; 6.5% would pursue neoadjuvant therapy, followed by resection of the primary or liver or both; and 2.2% would refer directly to a medical oncologist with deferral of recommendation.
Overall, surgeons felt that patients with sCRLM should be referred to CRS (63.0%), HPB surgeons (91.3%) and medical oncologists (84.8%). The presence of lung metastases altered this referral plan in 68.2% of respondents. Specifically, referral to a thoracic surgeon was recommended as the final step (75.0%), first step (12.5%), prior to any referral to medical oncology (10.0%), and prior to HPB (2.2%).
Operative management
The majority (63.5%) of respondents had the option/invitation of joining an HPB surgeon to perform concurrent resections. Respondents’ willingness to perform a combination of colonic and liver resections varied substantially depending on the magnitude of the procedure (Table 1). Most (88.9%) surgeons considered performing the first stage of a planned 2-stage hepatectomy with a simultaneous resection of the colorectal primary. Respondents suggested they would protect their anastomoses with a diverting stoma occasionally (56.1%), rarely (26.8%) and often (17.1%).
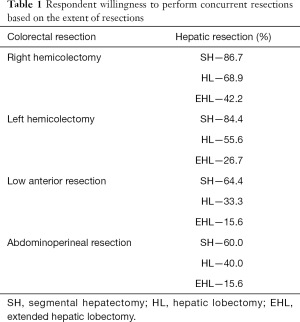
Full table
Simultaneous colorectal and hepatic resections were stated to be potentially beneficial in 61.7% of cases with a symptomatic primary cancer, whereas a preoperative colonic stent (57.4%) and neoadjuvant chemotherapy (55.3%) were considered preferable options for other respondents. While the vast majority (88.9%) of surgeons commented that the sequencing of hepatic and colorectal resections had changed in recent years, a large proportion (44.4%) believed they could still determine resectability of the hepatic lesions. In contrast, although the majority (87.0%) of respondents also commented that sCRLM and pulmonary metastases are potentially resectable, only 19.6% believed that they could determine the pulmonary component of resectability. The recommended sequencing of colon, liver and lung lesions was: colon and liver together, followed by lung (53.5%); colon, then liver, then lung (32.6%); and liver, then colon, then lung (11.5%); lung, then colon, then liver (2.3%).
Outcomes
The majority (77.8%) of respondents believed that simultaneous colorectal and hepatic resections carried an increased risk of morbidity, while 44.2% responded that they also carried a higher risk of mortality. Only 54.3% of surgeons felt that some patients would benefit from a liver-first approach with resection, followed by the colorectal primary. Finally, 23.8% of respondents reported awareness of evidence based guidelines and/or consensus statements directing the management of sCRLM.
CRS
Similar to the overall surgical group, CRS reported excellent working relationships with HPB surgeons (100%) and medical oncologists (95.8%). Most CRS (75.0%) believed that referral to HPB surgeons and medical oncologists of patients with asymptomatic or minimally symptomatic primary tumors and sCRLM was appropriate (vs. 59.1% in non-CRS; P=0.08). Most CRS (92.9%) also believed there was a role for simultaneous resection of primary and hepatic lesions (vs. 100% in non-CRS), and more (67.9%) had also participated in concurrent resections than non-CRS (41.7%; P<0.01). Compared with non-CRS, CRS displayed altered referral tendencies with regard to HPB surgeons in cases of symptomatic primaries, referral to colorectal specialists in cases of sCRLM, the importance of portal lymphadenopathy, patient comorbidities and the accessibility of HPB surgeons (Table 2). CRS’ referral patterns were less likely to be influenced by the presence of lung metastases (50.0% vs. 90.0%, P<0.01) but more likely to consider patients with lung metastases potentially curable (100.0% vs. 72.7%, P<0.01).
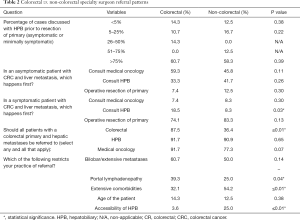
Full table
Discussion surrounding specific combinations of simultaneous colorectal and hepatic resections showed that CRS are willing to be more aggressive with reference to extended hepatectomies, low anterior resections, abdominal-perineal resections, and fewer protective diverting stomas (Table 3). Although a large portion (37.5%) of CRS believed they could determine hepatic lesion resectability (vs. 52.4% for non-colorectal), all believed HPB surgeons were the best suited to truly determine resectability. Similar to non-CRS, most CRS (91.7%) believed that neoadjuvant chemotherapy prior to surgical intervention could be helpful (vs. 77.3%; P=0.07), and were not familiar (29.2%) with consensus based guidelines on this topic (vs. 27.3% in non-CRS).
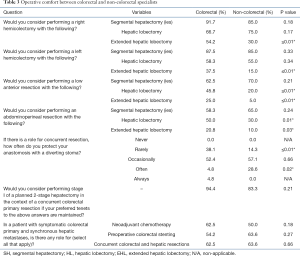
Full table
Although all surgeons believed that simultaneous resections carried a higher risk of morbidity (83.3% vs. 71.4%; P=0.13), CRS also believed they carried a higher risk of mortality (56.5% vs. 30.0%; P<0.01).
Discussion
The surgical treatment for sCRLM has improved substantially over the past 15 years. This evolution is a direct result of significantly more efficacious systemic chemotherapy, better individualization of surgical/medical care, and a tremendous improvement in the safety and quality of hepatic resection. Despite these advances, there remains a perception that significant heterogeneity exists with respect to both referral patterns and surgeon beliefs surrounding simultaneous colorectal and hepatic resections. To this end, the aim of this survey was to better define these two concepts within the colorectal and general surgical communities.
The surgical literature describing the management of patients with sCRLM supports the concept of simultaneous resections with a number of caveats. More specifically, it is clear that in properly selected patients, simultaneous resections for sCRLM can be performed with comparable morbidity, mortality, and long-term oncologic outcomes when compared to stage resections. This viewpoint is best represented in a large, multi-institutional study reporting on 1,004 patients treated for sCRLM (6). More specifically, the complication rates (20%), 90-day mortality (3.0%) and long-term survival (median =51 months; 5-year =44%) was statistically similar between staged and simultaneous resections. An even more recent analysis using the American College of Surgeons NSQIP (ACS-NSQIP) described 43,408 patients who underwent an isolated hepatectomy, isolated colorectal resection, or simultaneous resection concluded that the major morbidity after concurrent resections varies incrementally with the risk of the individual procedures (5). This is especially true regarding the magnitude of the hepatectomy (5). Although this study was not powered to make granular conclusions surrounding simultaneous major hepatectomies, it did appear that ‘minor’ hepatectomies were low risk in the context of both low and high risk colorectal resections. As one would expect, it is also apparent that with increasing blood loss and prolonged operative times, the risk of anastomotic leakage from a gastrointestinal anastomosis increases (8). These two quality metrics are known to have the same effect on the morbidity and mortality of numerous complex surgical procedures (9).
The dominant finding in this Canadian survey is the tremendous heterogeneity in both surgeon referral patterns, as well as specific beliefs surrounding simultaneous resections of sCRLM. More specifically, although access to HPB surgeons and medical oncologists was reported as excellent overall (94% and 100% respectively), a large proportion of surgeons do not typically discuss patients with sCRLM prior to their operative intervention on the primary lesion. Only 60% of surgeons regularly discuss these cases with HPB surgeons (i.e., more than 75% of their synchronous cases) in the preoperative setting. This is particularly interesting given that 94% of surgeons reported they believe there is a role for simultaneous resections. It is also not surprising that surgeons from smaller hospitals (less inpatient beds and smaller referral volumes) reported less frequently having ‘easy’ access within their institution to an HPB surgeon. This observation is expected as HPB care is almost exclusively centralized into high volume academic institutions within Canada. It was also interesting to note that survey response differences were more frequently highlighted by variances between surgeon subspecialty (CRS vs. non-colorectal) as opposed to institutional size.
Upon a more detailed case-based analysis, a significant difference in referral patterns was noted based on the symptoms associated with the primary tumor. More surgeons would resect the primary lesion prior to consultation in patients with a symptomatic tumor (78%) compared to an asymptomatic lesion (10%). Interestingly, as a group, the CRS (18.5%) would refer patients with symptomatic colorectal primaries to an HPB surgeon more frequently than non-CRS (8.3%). While the magnitude of physiologic derangement associated with a near complete bowel obstruction for example would clearly prohibit any form of simultaneous resection, more subtle symptoms (low quantity bleeding, partial obstructions, mild discomfort) would not necessarily preclude this option. It also commonly understood that in some patients, neoadjuvant systemic therapy is preferable and treats not only the hepatic metastases, but also the primary tumor. Not surprisingly, the majority of surgeons (53%) would refer their asymptomatic patients to a medical oncologist first. Similarly, as a concept, the majority (75%) of CRS believed that referral of asymptomatic or minimally symptomatic patients to an HPB surgeon or medical oncologist was preferable. This referral pattern is becoming increasingly important given the known improvements in our understanding of the biology of sCRLM. More specifically, it is clear that some patients benefit from a “reverse strategy” (preoperative chemotherapy followed by resection of the liver metastases and then by resection of the colorectal primary at a second operation) (10). As with the timing of systemic chemotherapy, the sequencing of colorectal and hepatic resections should be individualized based on the specific patient and tumor characteristics in each case scenario. For example, a severely obstructed patient would benefit from the “classic strategy” of a colorectal resection followed by systemic therapy, then a hepatic resection. Other patients excel in the setting of simultaneous resections.
Upon deeper questioning regarding the potential reasons for restricting referral to an HPB surgeon, themes such as (I) bilobar hepatic metastases, (II) portal lymphadenopathy, (III) patient comorbidities, (IV) age, and (V) limitations in easy access to HPB surgeons were common. This list of limitations is particularly interesting given many of the outdated concepts. With the advent of safer hepatic resections (11,12), improved neoadjuvant chemotherapy (13,14), liver hypertrophy techniques portal vein embolization (15), hepatic arterial embolization, and associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) (16), two-stage hepatectomy strategies (17-19), and the ability to combine resections with percutaneous therapies [ablation (radiofrequency and microwave), chemoembolization (Transcatheter Arterial Chemoembolization—TACE) (20), radioembolization (yttrium-90)] (21) and/or hepatic arterial chemotherapy infusion (22), many patients with bilobar disease are resectable and treatable at all lesion sites. Similarly, the presence of enlarged lymph nodes within the porta hepatis does not represent an absolute contraindication to resection, nor do patient ages or comorbidities. Many of these patient factors require input from a sophisticated preoperative assessment engaging both internal medicine and anesthesia specialists who are well versed in the physiologic challenges associated with hepatic resection. In summary, employing this potentially outdated list of contraindications would clearly lead to the exclusion of some patients with resectable disease from potentially curative surgery.
The addition of lung metastases to the case scenario was interesting, as the specific position of the thoracic surgeon within the referral process was heterogeneously reported. More specifically, the majority (68%) of surgeons believed the presence of lung metastases would alter their referral plan. This is certainly appropriate given the inherent complexity in framing both synchronous indeterminate pulmonary nodules and the potentially improved survival noted after resection of both liver and lung colorectal metastases (23,24). Furthermore, only 20% of surgeons believed they could determine resectability of the pulmonary lesions. This is notably lower than the 44% overall, and 38% of CRS who believed they could determine the resectability of hepatic metastases. Given the inherent complexity and multimodality therapies described above for hepatic treatment, this high level of confidence in determining resectability is surprising and warrants further blinded investigation.
It is also potentially concerning that a large observed proportion of surgeons (44%) reported that they associated simultaneous resections with higher mortality (both CRS and non-CRS). As noted above, this belief has not shown to bear out in appropriately selected patients. If this is a true rationale for limiting consideration of simultaneous resections, patients may potentially miss out on the benefits of a combined approach (25). More specifically, simultaneous resections have been shown to be oncologically equivalent and more cost effective (7). They also display a reduction in overall length of hospital stay, and at our own institution (Calgary), a lowered total duration of treatment from 48 to 30 weeks. This benefit of reducing the overall treatment time cannot be underestimated with regard to both the physical and psychological status of a patient, as well as their financial/return to work challenges.
Numerous differences were reported in surgeon beliefs surrounding sCRLM between CR and non-colorectal specialists. CRS commented that they more commonly (88%) believed a patient with synchronous disease, in the context of a non-symptomatic primary tumor, should be referred to a CRS (vs. 36% in non-CRS). Although we were not able to definitively explain this difference, we suspect it relates to the more common location of CRS in centers with HPB expertise and therefore more options for simultaneous treatment. Similarly, CRS were also willing to be more surgically aggressive in the simultaneous operations themselves when compared to non-CRS. More specifically, CRS were more often willing to engage in hepatic lobectomies or extended lobectomies in the setting of low anterior rectal resections. Although the pattern of less tolerance of synchronous hepatic surgical risk as the colorectal primary cancer moved distally was also consistent amongst the CRS, their absolute level of tolerance was higher when compared to the non-CRS. This is likely a reflection of the more commonly reported first hand involvement amongst CRS in these simultaneous cases.
Limitations of this survey-based study include: (I) the inability to accurately define the response rate. As described, this survey was offered to members of the CAGS and CSCRS. While each of these organizations maintains a significant membership, the survey was not mandatory and therefore it remains unclear how many actual invitees received the notification. As a result, the possibility of a response bias cannot be eliminated; (II) a reasonably high number of respondents (53.8%) reported access to HPB surgeons at their own institution. This represents a potential bias towards an academic colorectal and general surgical practice. As a result, this survey represents a nearly 50/50 split between more academic versus community surgical practices; and (III) any survey that incorporates case-based scenarios to determine subsequent care will naturally lack some detail given the format. While it is unlikely that this lack of granular fidelity would alter responses, it remains possible.
Conclusions
In conclusion, this survey provides insight into many surgeon beliefs surrounding simultaneous resections for sCRLM. Assuming that a patient centered approach aiming for cure is the dominant goal for all clinicians, and then each of our subspecialty groups should strive for improvement. Hepatobiliary surgeons should enhance upon their ease of accessibility, as well as their reported 36% limitation to referring surgeons joining them during simultaneous resections. CRS have opportunities for improvement by increasing the proportion of patients who are referred to HPB surgeons for consideration of resection given the tremendous advancements in treatment options and safety for hepatic lesions. Non-CRS might consider earlier referral of their asymptomatic and minimally symptomatic patients to multidisciplinary discussions that include HPB surgeons, medical oncologists and potentially CRS with the expectation that their involvement in these cases will remain intact. The power of multidisciplinary case conference review in particular has become evident (26). Only through continued improvement in our collaboration will we be able to define the treatment sequence that is best tailored to a given patient.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the University of Calgary Conjoint Research Ethics Board (No. HREBA.CC-14-0170).
References
- Edwards BK, Noone AM, Mariotto AB, et al. Annual Report to the Nation on the status of cancer, 1975-2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer 2014;120:1290-314. [Crossref] [PubMed]
- Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin 2014;64:104-17. [Crossref] [PubMed]
- Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2011. Available online: http://cancer.gov/csr/1975_2011/
- Manfredi S, Lepage C, Hatem C, et al. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg 2006;244:254-9. [Crossref] [PubMed]
- Shubert CR, Habermann EB, Bergquist JR, et al. A NSQIP Review of Major Morbidity and Mortality of Synchronous Liver Resection for Colorectal Metastasis Stratified by Extent of Liver Resection and Type of Colorectal Resection. J Gastrointest Surg 2015;19:1982-94. [Crossref] [PubMed]
- Mayo SC, Pulitano C, Marques H, et al. Surgical management of patients with synchronous colorectal liver metastasis: a multicenter international analysis. J Am Coll Surg 2013;216:707-16; discussion 716-8. [Crossref] [PubMed]
- Abbott DE, Cantor SB, Hu CY, et al. Optimizing clinical and economic outcomes of surgical therapy for patients with colorectal cancer and synchronous liver metastases. J Am Coll Surg 2012;215:262-70. [Crossref] [PubMed]
- Nakajima K, Takahashi S, Saito N, et al. Predictive factors for anastomotic leakage after simultaneous resection of synchronous colorectal liver metastasis. J Gastrointest Surg 2012;16:821-7. [Crossref] [PubMed]
- Ball CG, Pitt HA, Kilbane ME, et al. Peri-operative blood transfusion and operative time are quality indicators for pancreatoduodenectomy. HPB (Oxford) 2010;12:465-71. [Crossref] [PubMed]
- Brouquet A, Mortenson MM, Vauthey JN, et al. Surgical strategies for synchronous colorectal liver metastases in 156 consecutive patients: classic, combined or reverse strategy? J Am Coll Surg 2010;210:934-41. [Crossref] [PubMed]
- Helton WS. SSAT/AHPBA Joint Symposium: Today's approaches to colorectal cancer (CRC) liver metastases. J Gastrointest Surg 2011;15:404-5. [Crossref] [PubMed]
- Ali MA, Di Sandro S, Lauterio A, et al. Repeat Hepatectomy for Recurrent Colorectal Liver Metastases: Is it Worth the Challenge? J Gastrointest Surg 2015;19:2192-8. [Crossref] [PubMed]
- Chan G, Hassanain M, Chaudhury P, et al. Pathological response grade of colorectal liver metastases treated with neoadjuvant chemotherapy. HPB (Oxford) 2010;12:277-84. [Crossref] [PubMed]
- Giuliante F, Ardito F, Ferrero A, et al. Tumor progression during preoperative chemotherapy predicts failure to complete 2-stage hepatectomy for colorectal liver metastases: results of an Italian multicenter analysis of 130 patients. J Am Coll Surg 2014;219:285-94. [Crossref] [PubMed]
- Shindoh J, Tzeng CW, Aloia TA, et al. Portal vein embolization improves rate of resection of extensive colorectal liver metastases without worsening survival. Br J Surg 2013;100:1777-83. [Crossref] [PubMed]
- Schadde E, Raptis DA, Schnitzbauer AA, et al. Prediction of Mortality After ALPPS Stage-1: An Analysis of 320 Patients From the International ALPPS Registry. Ann Surg 2015;262:780-5; discussion 785-6. [Crossref] [PubMed]
- Tsai S, Marques HP, de Jong MC, et al. Two-stage strategy for patients with extensive bilateral colorectal liver metastases. HPB (Oxford) 2010;12:262-9. [Crossref] [PubMed]
- Lam VW, Laurence JM, Johnston E, et al. A systematic review of two-stage hepatectomy in patients with initially unresectable colorectal liver metastases. HPB (Oxford) 2013;15:483-91. [Crossref] [PubMed]
- Schadde E, Slankamenac K, Breitenstein S, et al. Are two-stage hepatectomies associated with more complications than one-stage procedures? HPB (Oxford) 2013;15:411-7. [Crossref] [PubMed]
- Massmann A, Rodt T, Marquardt S, et al. Transarterial chemoembolization (TACE) for colorectal liver metastases--current status and critical review. Langenbecks Arch Surg 2015;400:641-59. [Crossref] [PubMed]
- Abbott AM, Kim R, Hoffe SE, et al. Outcomes of Therasphere Radioembolization for Colorectal Metastases. Clin Colorectal Cancer 2015;14:146-53. [Crossref] [PubMed]
- Kemeny NE, Chou JF, Boucher TM, et al. Updated long-term survival for patients with metastatic colorectal caner treated with liver resection followed by hepatic arterial infusion and systemic chemotherapy. J Surg Oncol 2016;113:477-84. [Crossref] [PubMed]
- Gomez D, Kamali D, Dunn WK, et al. Outcomes in patients with indeterminate pulmonary nodules undergoing resection for colorectal liver metastases. HPB (Oxford) 2012;14:448-54. [Crossref] [PubMed]
- Brouquet A, Vauthey JN, Contreras CM, et al. Improved survival after resection of liver and lung colorectal metastases compared with liver-only metastases: a study of 112 patients with limited lung metastatic disease. J Am Coll Surg 2011;213:62-9. [Crossref] [PubMed]
- Schwarz RE, Abdalla EK, Aloia TA, et al. AHPBA/SSO/SSAT sponsored consensus conference on the multidisciplinary treatment of colorectal cancer metastases. HPB (Oxford) 2013;15:89-90. [Crossref] [PubMed]
- Wanis KN, Pineda-Solis K, Tun-Abraham ME, et al. Management of colorectal cancer with synchronous liver metastases: impact of multidisciplinary case conference review. Hepatobiliary Surg Nutr 2017;6:162-9. [Crossref] [PubMed]


