Astrocyte elevated gene-1 (AEG-1): a new potential therapeutic target for the treatment of nonalcoholic steatohepatitis (NASH)
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease worldwide, which affects 25% of the general adult population and 70–80% of individuals with obesity and diabetes (1). In Asia, the prevalence of NAFLD is in the range of 15% to 30% in the general population and over 50% in patients with diabetes and metabolic syndrome (2). A disease spectrum of NAFLD ranges from simple steatosis to steatosis with liver inflammation and fibrosis, referred to as nonalcoholic steatohepatitis (NASH). Up to 30% of NAFLD patients have NASH and approximately 41% of NASH patients experience fibrosis progression which significantly increases the risk of hepatocellular carcinoma (1). Treatment for NAFLD and NASH is challenging. There are currently no medicines approved by the United States Food and Drug administration for the treatment of these diseases. Therefore, a better understanding of the molecular pathogenesis of NASH is urgently needed to develop effective therapeutic strategies.
Astrocyte elevated gene-1 (AEG-1), also known as metadherin (MTDH) and LYRIC, is a multifunctional oncogene which is overexpressed in nearly all tumor types. AEG-1 plays an important role in regulating tumor cell proliferation, invasion, and metastasis. AEG-1 is also associated with angiogenesis, chemoresistance, and protection from apoptosis (3). In a recent article published in Hepatology by Srivastava et al. entitled “A novel role of Astrocyte Elevated Gene-1 (AEG-1) in regulating nonalcoholic steatohepatitis (NASH)” (4), the investigators discovered that AEG-1 is an important regulator in regulating the development of NASH. They generated a transgenic mouse with hepatocyte-specific overexpression of AEG-1 (Alb/AEG-1) and investigated its oncogenic functions (5). Interestingly, they discovered that Alb/AEG-1 mice spontaneously developed NASH, suggesting that AEG-1 prevents the spontaneous development of NASH. Subsequently, the underlying mechanisms of AEG-1 in regulating NASH were investigated in this study.
First, the authors confirmed that Alb/AEG-1 mice develop spontaneous NASH, along with increased fat droplets, fibrosis, and inflammation in the liver in Alb/AEG-1 mice compared to WT mice. AEG-1 overexpression also exacerbated steatotic phenotypes when mice were fed a high fat diet (HFD) for 20 weeks. The hepatic levels of AEG-1 were increased in the mice fed with a HFD as well as in human NASH patients. These findings strongly suggest that AEG-1 overexpression in the liver is associated with the development of NASH.
To investigate the role of AEG-1 endogenously expressed in hepatocytes in NASH, the investigators generated a hepatocyte-specific conditional AEG-1 knockout mouse (AEG-1ΔHEP) model. AEG-1ΔHEP mice showed decreased fat deposition, fibrosis, and inflammation in the liver, demonstrating that AEG-1ΔHEP mice were protected from HFD-induced NASH and suggesting that AEG-1 is required for the initiation and progression of NASH.
What are the mechanisms of AEG-1 in regulating NASH? The investigators compared the gene expression profiles in hepatocytes between WT and Alb/AEG-1 mice using microarray analysis. AEG-1-overexpressed hepatocytes showed a robust inhibition in the genes regulated by peroxisome proliferator-activated receptor (PPAR)α, PPARγ and PXR, and the robust activation in genes involved in inflammatory signaling such as Toll-like receptor (TLR)-4, TLR-3, IL-1β, tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6) and NF-κB complex. RNA sequencing (RNA-Seq) analysis results showed that expression of PPARα and PPARγ was upregulated and expression of TNF-α was downregulated in AEG-1ΔHEP mice, which corroborates the Alb/AEG-1 mice findings.
These gene expression profiles suggest that AEG-1 was important in regulating the PPARα pathway. Thus, the investigators focused on AEG-1-mediated regulation of PPARα activity. PPARα is a ligand-activated nuclear receptor highly expressed in the liver, which is involved in peroxisomal and mitochondrial β-oxidation, fatty acid (FA) transport, and hepatic glucose production (6). In this study, PPAR response element (PPRE) luciferase (luc) activity was examined in WT and AEG-1-overexpressed hepatocytes. The PPRE-luc activity was reduced in AEG-1-overexpressed hepatocytes compared to WT cells. The expression of PPARα and its coactivator PPARγ coactivator 1 alpha (PGC1α) was downregulated in AEG-1-overexpressed hepatocytes. The study also determined the decreased expression of PPARα target genes that regulate FA β-oxidation, such as carnitine palmitoyltransferase 1a (Cpt1a), acylCoA dehydrogenase (Acad) l, and Acadm in AEG-1-overexpressed hepatocytes. In contrast, these genes were increased in AEG-1-/- hepatocytes. The hepatic FA β-oxidation was significantly suppressed in AEG-1-overexpressed mice. Moreover, AEG-1 overexpression suppressed the recruitment of RXRα/PPARα heterodimer and coactivator steroid receptor coactivator-1 (SRC-1) on Acox1 and Cpt1a promoter region. These results suggest that AEG-1 can suppress PPARα activity, thereby decreasing FA β-oxidation.
Their gene expression profiles also suggested that AEG-1 promotes inflammatory signaling. A significant increase in mRNA levels of inflammatory cytokines, such as IL-1β, IL-6 and TNF-α, were observed in livers of chow-fed Alb/AEG-1 mice compared to WT mice, which was further augmented when mice were fed HFD. Protein levels of TNF-α and phosphorylated NF-κBp65 were increased in hepatocytes from Alb/AEG-1 mice while decreased in livers from AEG-1ΔHEP mice. On the other hand, the authors also found that TNF-α and IL-1β treatment significantly upregulates AEG-1 mRNA expression in human and mouse hepatocytes. Since AEG-1 expression was inhibited by an inhibitor of IκB kinase (IKK), NF-κB plays an important role in TNF-α and IL-1β-mediated AEG-1 upregulation. These findings demonstrated that NF-κB and AEG-1 reciprocally regulate, which sustain chronic inflammation in the liver.
Increased hepatic de novo lipogenesis, the synthesis of new FAs from non-lipid sources, is an important feature of NASH. Fatty acid synthase (Fasn) is a key enzyme for de novo lipogenesis, which is transcriptionally regulated by LXR. Activated LXR binds to LXR response element in the Fasn promoter. LXR also regulates sterol regulatory element-binding protein-1 (SREBP-1) that controls transcription of a number of lipogenesis-associated genes. There were no significant differences in LXR/RXR activity or Fasn mRNA levels among in WT, AEG-1-overexpressed, and AEG-1-deficient hepatocytes. These findings strongly suggested that AEG-1 does not affect LXR-mediated gene expression. However, interestingly, the protein levels of Fasn and other lipogenesis-related enzymes were significantly increased in AEG-1-overexpressed livers, which promote de novo lipogenesis. They also confirm it by analysis of lipid fractions. AEG-1 overexpression also assists Fasn mRNA to associate with polysomes, thereby promoting translation of Fasn protein. Moreover, AEG-1 overexpression accelerated and enhanced de novo Fasn protein synthesis. These findings suggest that AEG-1 promotes de novo lipogenesis, at least in part, by a translational regulation of Fasn.
RNA interference (RNAi) is a potent and specific approach to inhibit target gene expression. RNAi can be a useful strategy for treating liver diseases because intravenously injected payload will preferentially deliver to the liver. Lipid nanoparticles (LNP) have been shown to be highly effective in delivering siRNAs to the liver and silencing a number of different hepatocyte gene targets across multiple species. A phase I clinical trial using LNP-conjugated siRNA in patients with advanced cancer and liver metastases showed satisfactory regression of liver metastasis (7), which provides evidence for the use of nanoparticles in the treatment of liver diseases. The investigators developed liver-targeted nanoplexes by conjugating polyamidoamine (PAMAM) dendrimers with polyethylene glycol (PEG) and galactose lactobionic acid (PAMAM-PEG-Gal), which were combined with AEG-1 small interfering RNA (siRNA; PAMAMAEG-1si) and have documented its therapeutic effects in HCC previously (8). In this study, they further examined its efficacy in inhibiting HFD-mediated NAFLD. The results showed that PAMAM-AEG-1si treatment significantly suppressed HFD-mediated hepatic steatosis, inflammation, and fibrosis compared to PAMAM-siCon treatment, suggesting that targeting AEG-1 and using nanoparticles can be a potential effective approach for the treatment of NAFLD.
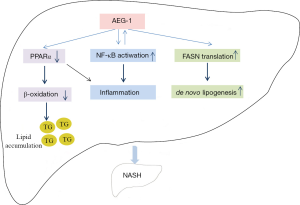
In conclusion, Srivastava et al. provided a new role of AEG-1 in regulating the development of NASH. The proposed mechanism of AEG-1 in promoting NASH could be mediated by decreased PPARα activity, enhanced translation of Fasn protein, and increased NF-κB-mediated inflammation (Figure 1). This suggests that inhibition of hepatic AEG-1 expression or activity can be a new effective strategy to halt NASH progression. Thus, AEG-1 could be a key molecule that regulates the development of NASH, and a potential therapeutic target for the treatment of NASH.
Acknowledgements
The authors thank Ms Daisy Gomez (Department of Medicine, Cedars-Sinai Medical Center) for editing the manuscript.
Funding: This work is supported by NIH grants R01DK085252 and R21AA025841, and Winnick Research award from Cedars-Sinai Medical Center (to Ekihiro Seki); Qinglan Wang is supported by National Natural Science Foundation of China (81573810, 81270053) and China Postdoctoral Science Foundation (2015T80445).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Lonardo A, Byrne CD, Caldwell SH, et al. Global epidemiology of nonalcoholic fatty liver disease: Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:1388-9. [Crossref] [PubMed]
- Wong VW. Nonalcoholic fatty liver disease in Asia: a story of growth. J Gastroenterol Hepatol 2013;28:18-23. [Crossref] [PubMed]
- Yoo BK, Emdad L, Lee SG, et al. Astrocyte elevated gene-1 (AEG-1): A multifunctional regulator of normal and abnormal physiology. Pharmacol Ther 2011;130:1-8. [Crossref] [PubMed]
- Srivastava J, Robertson CL, Ebeid K, et al. A novel role of astrocyte elevated gene-1 (AEG-1) in regulating nonalcoholic steatohepatitis (NASH). Hepatology 2017;66:466-80. [Crossref] [PubMed]
- Srivastava J, Siddiq A, Emdad L, et al. Astrocyte elevated gene-1 promotes hepatocarcinogenesis: novel insights from a mouse model. Hepatology 2012;56:1782-91. [Crossref] [PubMed]
- Chinetti G, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors (PPARs): nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflamm Res 2000;49:497-505. [Crossref] [PubMed]
- Tabernero J, Shapiro GI, LoRusso PM, et al. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov 2013;3:406-17. [Crossref] [PubMed]
- Rajasekaran D, Srivastava J, Ebeid K, et al. Combination of Nanoparticle-Delivered siRNA for Astrocyte Elevated Gene-1 (AEG-1) and All-trans Retinoic Acid (ATRA): An Effective Therapeutic Strategy for Hepatocellular Carcinoma (HCC). Bioconjug Chem 2015;26:1651-61. [Crossref] [PubMed]


