Bone morphogenetic protein-9/activin-like kinase 1 axis a new target for hepatic regeneration and fibrosis treatment in liver injury
Bone morphogenetic protein-9 (BMP-9), a member of the transforming growth factor (TGF)-β family of cytokines, is preferentially expressed in the liver from where is secreted into the blood (1-3). BMP-9 and BMP-10 are high-affinity ligands for the type I receptor activin-like kinase 1 (ALK1), whose participation in liver fibrosis has been previously suggested, and activin type II receptor A (ActRIIA) a BMP type II receptor with undefined role in liver biology. Although BMP-9 promotes epithelial to mesenchymal transition in hepatocellular carcinoma (HCC) cells, correlating with HCC aggressiveness in patients, and the BMP-9/ALK1 signaling has been related to liver pathologies, little was known about BMP-9 specific functions in liver cells and, particularly, after hepatic injury.
Filling this gap, Breitkopf-Heinlein et al. (4) have recently provided interesting clues of BMP-9 liver function and pointed to novel aspects that will merit future attention. In their Gut publication, the authors identify hepatic stellate cells (HSCs) as the cellular source for BMP-9 secretion with increasing amounts during fibrogenic activation. In addition to possible effects on other non-parenchymal liver cells that exhibit important levels of BMP-9 receptor ALK1, such as liver sinusoidal endothelial cells (LSECs) and Kupffer cells, BMP-9 autocrine action could increase HSC proliferation and migration as observed in vitro in cultured HSCs.
Moreover, BMP-9 stabilizes healthy hepatocytes helping them to maintain cell polarization and functionality. These anti-proliferative and anti-EMT activities observed in primary hepatocytes contrast with the EMT promoting action of BMP-9 on HCC cells previously detected, suggesting completely opposed functions for BMP-9 in tumoral and non-tumoral conditions. Not only that, BMP-9 would interfere with hepatic wound healing and regeneration after acute liver damage. To explain the apparent contradiction of BMP-9, preserving the physiological metabolic activity in the hepatocyte but enhancing liver damage after acute hepatic injury, BMP-9 has been proposed as a stabilizer of the parenchyma, useful under healthy conditions but problematical after damage when issues that must be further analyzed before its clinical application. Hepatocytes have to proliferate and cell plasticity is required. In fact, not only BMP-9 reduction will be required to facilitate hepatic recovery, but also it will be beneficial to lower BMP-9 levels to reduce fibrogenesis as shown in a CCl4-induced chronic liver damage mouse model.
Differences in the expression levels of the individual BMP-9 associated receptors and co-factors or in the BMP-9 interplay with other signaling pathways may explain these divergent behaviors, issues that must be further analyzed before its clinical application. In particular, some concerns may arise by anti-BMP-9/ALK1 therapies that could promote hepatocyte dedifferentiation and proliferation in individuals with chronic liver disease, where the inter-cellular environment with altered extracellular matrix and hepatic inflammation facilitates HCC development and metastasis implantation.
Regarding the canonical signaling triggered by BMP-9 binding, activated receptors interact with Smad1, Smad5, Smad8 and phosphorylate them. After nuclear translocation, the activated Smad complex binds to a Smad-responsive element and induce specific target gene such as the co-receptor Endoglin, Id1 or Snail-1. Of note, several publications have directly and indirectly related these proteins to liver fibrosis in different clinical and experimental settings (5-7), and now, their present connection with BMP-9 signaling may justify, at least partly, some of these results. In addition, it should not be forgotten that BMPs activate Smad-independent pathways in specific cell types. This non-canonical or non-Smad pathways includes MAPK (p38, ERK and JNK), PI3K/AKT, NF-κB, Wnt, or microRNA regulation, among others.
Another interesting issue of BMP-9/ALK1 signaling may be its participation in hepatic inflammation during chronic liver injury. Regarding this point, the authors have proposed that the reduction of BMP-9 expression after acute liver injury may be due to the LPS inhibitory effect on BMP-9 expression, as they observed in cultured HSCs. In a complementary approach, recent data points the BMP-9/ALK1 axis to enhance LPS-induced leukocyte recruitment by upregulating TLR4 expression in human endothelial cells (8). Since small interfering RNA knockdown ALK1 blocked this effect, both proteins BMP-9 and ALK1 are potential targets for therapeutic intervention. As Breitkopf-Heinlein et al. indicate, BMP-9 is increased in chronic liver disease and BMP-9/ALK-1 axis inhibition could reduce TLR4 signaling diminishing hepatic inflammation and neutrophil infiltration. This potential modulation of inflammatory processes in the liver justifies, per se, future studies in BMP-9 under conditions of chronic damage where the role played by other non-parenchymal cell lines, particularly LSECs and Kupffer cells, should be addressed. As also shown by the authors, Kupffer cells express high levels of BMP-9 receptor ALK1 and the effect of BMP-9 reduction in activated Kupffer cells could be highly relevant in the context of chronic liver disease.
Evidently, numerous beneficial effects are expected from BMP-9 action in non-pathological conditions (Figure 1) and tampering with BMP-9/ALK1 signaling for long-time periods, as probably required to obtain positive results in fibrosis resolution and hepatic recovery, may have unknown effects on human biology. In this sense, inhibitors of this pathway are already in clinical trials for anti-angiogenic therapy to cancer patients (9). PF-03446962, is a fully human antibody against the extracellular domain of ALK1 that inhibits all ALK1 signaling independently of being induced by BMP-9 or other BMPs, and Dalantercept/ACE-041, is an ALK1-Fc fusion protein that sequesters the ALK1 high affinity ligands BMP-9 and BMP-10, preserving ALK1 activation through other ligands. Both inhibitors have shown good tolerability in patients and are expected to be worthy additions for the anti-cancer arsenal, circumstance that will facilitate their quick transition to human testing in liver pathologies.
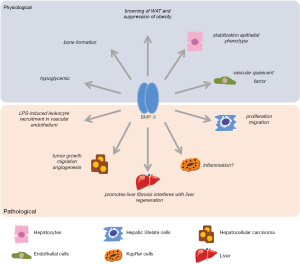
As the attention to liver fibrosis in general, and to NASH-induced fibrosis in particular has grown, a cascade of novel signaling pathways (10-13) has been recently related to this pathology. Joining this group, Breitkopf-Heinlein et al. and others publications have positioned the BMP-9/ALK1 axis as an interesting player in fibrosis and in liver regeneration. Obviously, additional knowledge of BMP-9 specific role on LSECs and Kupffer cells may be helpful, but we are convinced new data will soon be generated on this subject and it will help us better understand this novel and puzzling pathway.
Acknowledgements
Funding: The study was funded by grants from Instituto de Salud Carlos III (PI16/00930 to M Marí); Ministerio de Economía y Competitividad (SAF2015-66515-R to A Morales); co-funded by FEDER (Fondo Europeo de Desarrollo Regional, Unión Europea); and CERCA Programme/Generalitat de Catalunya.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Herrera B, Dooley S, Breitkopf-Heinlein K. Potential roles of bone morphogenetic protein (BMP)-9 in human liver diseases. Int J Mol Sci 2014;15:5199-220. [Crossref] [PubMed]
- Li W, Salmon RM, Jiang H, et al. Regulation of the ALK1 ligands, BMP9 and BMP10. Biochem Soc Trans 2016;44:1135-41. [Crossref] [PubMed]
- Bi J, Ge S. Potential roles of BMP9 in liver fibrosis. Int J Mol Sci 2014;15:20656-67. [Crossref] [PubMed]
- Breitkopf-Heinlein K, Meyer C, König C, et al. BMP-9 interferes with liver regeneration and promotes liver fibrosis. Gut 2017;66:939-54. [Crossref] [PubMed]
- Wiercinska E, Wickert L, Denecke B, et al. Id1 is a critical mediator in TGF-beta-induced transdifferentiation of rat hepatic stellate cells. Hepatology 2006;43:1032-41. [Crossref] [PubMed]
- Meurer SK, Tihaa L, Borkham-Kamphorst E, et al. Expression and functional analysis of endoglin in isolated liver cells and its involvement in fibrogenic Smad signalling. Cell Signal 2011;23:683-99. [Crossref] [PubMed]
- Rowe RG, Lin Y, Shimizu-Hirota R, et al. Hepatocyte-derived Snail1 propagates liver fibrosis progression. Mol Cell Biol 2011;31:2392-403. [Crossref] [PubMed]
- Appleby SL, Mitrofan CG, Crosby A, et al. Bone Morphogenetic Protein 9 Enhances Lipopolysaccharide-Induced Leukocyte Recruitment to the Vascular Endothelium. J Immunol 2016;197:3302-14. [Crossref] [PubMed]
- de Vinuesa AG, Bocci M, Pietras K, et al. Targeting tumour vasculature by inhibiting activin receptor-like kinase (ALK)1 function. Biochem Soc Trans 2016;44:1142-9. [Crossref] [PubMed]
- Marí M, Tutusaus A, García de Frutos P, et al. Genetic and clinical data reinforce the role of GAS6 and TAM receptors in liver fibrosis. J Hepatol 2016;64:983-4. [Crossref] [PubMed]
- Eguchi A, De Mollerat Du Jeu X, Johnson CD, et al. Liver Bid suppression for treatment of fibrosis associated with non-alcoholic steatohepatitis. J Hepatol 2016;64:699-707. [Crossref] [PubMed]
- Mridha AR, Wree A, Robertson AAB, et al. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. J Hepatol 2017;66:1037-46. [Crossref] [PubMed]
- Mukhopadhyay P, Horváth B, Rajesh M, et al. PARP inhibition protects against alcoholic and non-alcoholic steatohepatitis. J Hepatol 2017;66:589-600. [Crossref] [PubMed]


