A fermented mixed tea made with camellia (Camellia japonica) and third-crop green tea leaves prevents nonalcoholic steatohepatitis in Sprague-Dawley rats fed a high-fat and high-cholesterol diet
Introduction
Nonalcoholic fatty liver disease (NAFLD) is recognized as the most common cause of chronic liver disease in many countries. In most cases NAFLD does not progress beyond nonalcoholic fatty liver (NAFL), but the liver occasionally exhibits fibrosis and necroinflammation, indicating the presence of non-alcoholic steatohepatitis (NASH) (1). Based on the “two-hit” or “multiple parallel hits” hypothesis of NASH pathogenesis (2,3), insulin resistance, oxidative stress and activation of inflammatory cytokines seem to be key features in the transition from NAFL to NASH (4). Therefore, changes in dietary habits and lifestyle have been recommended as the standard of care for NAFLD; however, these behavioral strategies have a high failure rate (4).
Green tea (Camellia sinensis) is a popular beverage consumed worldwide. It has been recognized for its beneficial health effect such as hypocholesterolemic, hypotriacylglycerolemic, hypoglycemic, and antimutagenic activities (5). Further, it is rich in various polyphenols such as (−)-epigallocatechin-3-gallate, (−)-epigallocatechin, (−)-epicatechin-3-gallate, and (−)-epicatechin, which are thought to be the major compounds responsible for the above biological effects (5). Evidence from in vitro systems and animal models suggest that green tea catechins likely play a preventive role in hepatic steatosis by decreasing intestinal lipid and carbohydrate absorption, decreasing adipose lipolysis, stimulating hepatic β-oxidation and improving insulin sensitivity. Green tea catechins also prevent the progression to NASH through their antioxidant and anti-inflammatory properties (6). Li et al. reported that green tea extract protected against high fat-induced liver injury and NASH by lowering hepatic lipid accumulation and nuclear factor-kappa B (NF-κB)-dependent inflammation (7). However, Hirsch et al. recently reported that prolonged supplementation with green tea polyphenols exacerbated cholesterol-induced fatty liver by increasing hepatic oxidative stress and inflammation in mice (8). Teschke et al. also reported that green tea catechins were associated with the risk of hepatotoxicity such as drug-induced liver injury in human (9). Although green tea catechins inhibit the human hepatic and intestinal microsomal enzyme activity of various Cytochrome P450 (CYP) isofomes in vitro, there are no evidence that green tea catechins increase the risk of drug-induced liver injury (9,10).
Catechin oxidation in green tea leaves results in the formation of black tea polyphenols such as theaflavins and theasinensins. These polyphenols are reported to have hypocholesterolemic and hypotriacylglycerolemic effects in cholesterol-fed rats (11). Cai et al. reported that a fermented dark tea (Pu-erh tea) extract ameliorated high-fat diet-induced NASH by improving lipid metabolism, inflammation and insulin resistance (12).
Camellia japonica (Japanese camellia, named “tsubaki” in Japan) is one of the best known species of the genus Camellia. In the wild, it is found in mainland China, Taiwan, southern Korea and southern Japan. This camellia tree is widely grown in the Goto Islands, Nagasaki Prefecture, which are Japanese islands located in the East China Sea, off the western coast of Kyushu island; however, its leaves are not effectively utilized (13). “Tsubaki” tea (Camellia japonica) has beneficial effects on hepatic fat metabolism as with green tea, especially its lowering effect of hepatic fatty acid synthase activity, but its taste is not so good due to the astringent leaf flavor (13). Green tea cultivation is also popular in Nagasaki Prefecture, Japan. Green tea leaves are usually harvested several times per year at tea plantations. The first crop of green tea leaves is characterized by good taste and aroma. The third crop of green tea leaves contains a relatively large amount of catechins, which imparts a bitter taste, thereby diminishing the product’s commercial value (5,14). Thus, these two leaves are not effectively utilized and are in part discarded. Recently, fermented mixed tea (FMT) made with camellia and third-crop green tea leaves at a ratio of 1:9 by tea-rolling processing, named “Goto Tsubaki-cha”, have been commercialized (13). Tamaru et al. reported that this mixed tea contained black tea polyphenols such as theaflavins and theasinensins in addition to catechins, and that rats fed an extract of this mixed tea had significantly lower body weight and adipose tissue weight compared to rats fed camellia tea or third-crop green tea extract alone (15). Because visceral fat is one of the most important factors for the development of hepatic steatosis, this FMT supplementation can be a candidate dietary strategy for the prevention of NAFL or NASH.
In the present study, we evaluated the preventive effect of this FMT against NASH in Sprague-Dawley (SD) rats fed a high-fat and high-cholesterol (HFC) diet. We also investigated the mechanism underlying the effects of the FMT, specifically focusing on alterations in fibrogenesis, inflammation, oxidative stress and cholesterol or lipid metabolism in the liver.
Methods
Animals and experimental design
Eight-week-old male SD rats were purchased from Japan SLC (Hamamatsu, Japan) and housed individually in a temperature- and humidity-controlled room (22–24 °C and 50–60% relative humidity) with a 12-h light/dark cycle. After 1 week of acclimation with standard rodent chow (MF; Oriental Yeast, Tokyo, Japan) and water ad libitum, the rats were randomly divided into five groups: Control group (n=6), fed standard rodent chow (MF) as the normal diet for 9 weeks; HFC group (n=6), fed an HFC diet for 9 weeks; FMT1:5 group (n=7), fed a HFC diet supplemented with 1% (w/w) FMT (FMT) made with camellia (Camellia japonica) and third-crop green tea leaves at a ratio of 1:5 for 9 weeks; FMT1:2 group (n=6), fed a HFC diet supplemented with 1% (w/w) FMT at a ratio of 1:2 for 9 weeks; and FMT1:1 group (n=7), fed a HFC diet supplemented with 1% FMT at a ratio of 1:1 for 9 weeks. The HFC diet was prepared by mixing the MF with 30% (w/w) palm oil, 1.25% (w/w) cholesterol, and 0.5% (w/w) sodium cholate (16,17). FMT were provided by the Nagasaki Prefectural Agricultural and Forestry Experiment Station, Higashisonogi Tea Branch, Nagasaki, Japan, as described previously (15). The proximate dietary compositions of each diet fed to rats are shown in Table 1. Daily energy intake and body weight were monitored throughout the study.
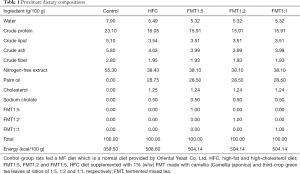
Full table
At 18 weeks of age, the rats were fasted for 8 h and sacrificed under anesthesia with pentobarbital sodium. Organs were harvested and blood was collected from the inferior vena cava. Liver tissues were either placed in 10% neutral buffered formalin or snap frozen in liquid nitrogen and stored at −80 °C.
Histopathological assessment of the liver
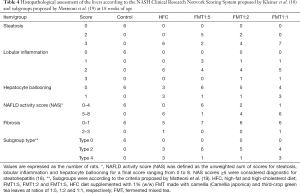
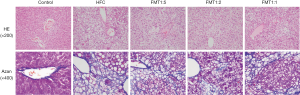
Liver tissues stored in 10% neutral-buffered formalin were embedded in paraffin, sectioned at 4 µm, and processed for hematoxylin-eosin (HE) staining for histopathological examination. Histological steatosis [0–3], lobular inflammation [0–3] and hepatocyte ballooning [0–2] were assessed semiquantitatively to determine the NAFLD activity score (NAS) according to the criteria proposed by Kleiner et al. (18). The final NAS values ranged from 0 to 8. NAS scores ≥5 and ≤2 were considered diagnostic and not diagnostic, respectively, for steatohepatitis. Liver fibrosis [0–4] was also assessed by Azan staining. Each specimen was also assigned to one of four histopathological subgroups: type 1, fatty liver alone; type 2, fat accumulation and lobular inflammation; type 3, fat accumulation and ballooning degeneration; type 4, fat accumulation, ballooning degeneration, and either Mallory hyaline or fibrosis, according to the criteria proposed by Matteoni et al. (19). All histopathological examinations were performed by a pathologist (K.T.) who was blinded to the experimental and serological data.
Serum biochemical analysis
Serum triglyceride (TG), total cholesterol (TC), glucose, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) were determined by Triglyceride E test Wako, Cholesterol E test Wako, Glucose C II test Wako, and Transaminase C II test Wako (Wako Pure Chemical Industries, Osaka, Japan), respectively. Serum insulin, leptin, and adiponectin levels were measured using a rat insulin enzyme-linked immunosorbent assay (ELISA) kit (Morinaga Institute of Biological Science Inc., Yokohama, Japan), a mouse/rat leptin ELISA kit (Morinaga Institute of Biological Science Inc.), and a mouse/rat adiponectin ELISA kit (Otsuka Pharmaceuticals Co., Ltd., Tokyo, Japan), respectively.
Quantification of mRNA using real-time PCR
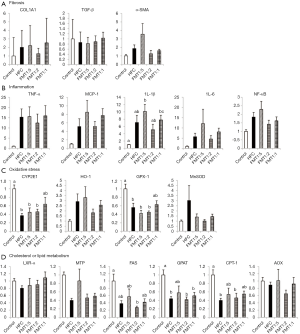
Total RNA from the liver was extracted using RNAiso Plus (Takara Bio, Otsu, Japan) according to the manufacturer’s instructions. RNA was reverse-transcribed to cDNA templates using a commercial kit (PrimeScript RT Master Mix, Takara Bio). Real-time PCR analysis was performed as described previously (16,17). Specific primers were designed using the primer designing tool Primer-BLAST [National Center for Biotechnology Information (NCBI), Bethesda, MD, USA] and were synthesized by Greiner Bio-One Japan (Tokyo, Japan; Table 2). mRNA expression was normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as a house-keeping gene. For studies in rats, hepatic expressions of genes involved in fibrosis [collagen type I alpha 1 (COL1A1), transforming growth factor-β (TGF-β), and α-smooth muscle actin (α-SMA)], inflammation [tumor necrosis factor-α (TNF-α), monocyte chemoattractant protein-1 (MCP-1), interleukin-1β (IL-1β), interleukin-6 (IL-6), and nuclear factor-κB (NF-κB)], oxidative stress [cytochrome P450 family 2 subfamily E polypeptide 1 (CYP2E1), heme oxygenase-1 (HO-1), glutathione peroxidase-1 (GPX-1), and manganese superoxide dismutase (MnSOD)], cholesterol metabolism [liver X receptor-α (LXR-α)], and lipid metabolism [microsomal triglyceride transfer protein (MTP), fatty acid synthase (FAS), glycerol-3-phosphate acyltransferase (GPAT), carnitine palmitoyltransferase-1 (CPT-1), and acyl CoA oxidase (AOX)] were quantified. All data were expressed as fold changes compared to Control group expression.

Full table
Statistical analysis
All values were expressed as mean ± standard error (SE). Differences between groups were tested for statistical significance using one-way analysis of variance (ANOVA), followed by Scheffe’s post hoc test, chi-square test, or Fisher’s exact probability test. All analyses were performed using IBM SPSS statistics software program, version 23 (IBM Co., Somers, NY, USA) on a Windows computer. A P value of less than 0.05 was considered to be statistically significant.
Results
Cumulative energy intake, body weight and relative organ weight
Cumulative energy intake was significantly higher in the HFC, FMT1:5, FMT1:2 and FMT1:1 groups than the Control group (P<0.01), whereas body weight at 18 weeks of age, weight gain during the 9-week study and food efficacy were not significantly different among the groups. The liver weight/body weight ratio at 18 weeks of age was significantly higher in the HFC, FMT1:5, FMT1:2 and FMT1:1 groups than the Control group (P<0.01), and this ratio was significantly lower in the FMT1:1 group than the HFC and FMT1:5 groups (P<0.01). The epididymal fat pad weight/body weight ratio was not significantly different among the groups (Table 3).

Full table
Histopathological findings of the liver
Representative histopathology of the rat liver and histological assessments is shown in Figure 1, Table 4 and Figure S1. No obvious findings of hepatic steatosis, lobular inflammation, hepatocyte ballooning or fibrosis were seen in any of the rats in the Control group. In contrast, severe steatosis (score 3) and moderate lobular inflammation were observed in all six rats of the HFC group. Hepatocyte ballooning and portal/periportal or bridging fibrosis (score 2 or 3) was observed in three and one of the six rats in the HFC group, respectively. In the FMT1:5 group, moderate steatosis (score 2), mild lobular inflammation (score 1) and no hepatocyte ballooning (score 0) was observed in 5 (71%), 3 (43%) and 6 (86%) of seven rats, respectively. In the FMT1:2 group, moderate steatosis (score 2), mild lobular inflammation (score 1) and no hepatocyte ballooning (score 0) was observed in 2 (33%), 1 (17%) and 5 (83%) of six rats, respectively. No portal/periportal or bridging fibrosis (score 2 or 3) was seen in any of the rats in the FMT1:5 and FMT1:2 groups. In the FMT1:1 group, the results of hepatic steatosis, lobular inflammation, hepatocyte ballooning or fibrosis were similar to those in the HFC group. According to the NAS (18), none of six rats (0%), all of six rats (100%), one of seven rats (14%), four of six rats (67%) and six of seven rats (86%) in the Control, HFC, FMT1:5, FMT1:2 and FMT1:1 groups, respectively, were diagnosed with NASH (i.e., having a NAS of 5 or greater). According to the criteria proposed by Matteoni et al. (19), three of six (50%) HFC group rats and three of seven (43%) FMT1:1 group rats were type 4, representing NASH, whereas one of seven (14%) FMT1:5 group rats and one of six (17%) FMT1:2 group rats had definitive evidence of NASH.
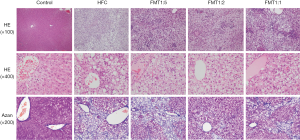
Full table
Serum biochemical parameters
Serum TG levels were not significantly different among the groups, whereas serum TC levels were significantly higher in the HFC group than the Control group (P=0.049) and were significantly lower in the FMT1:2 group than the HFC and FMT1:5 groups (P=0.016 and 0.049, respectively). Serum glucose levels did not significantly differ among the groups. Serum insulin and leptin levels both tended to be lower in the FMT groups in a green tea extract dose-dependent manner compared to the HFC group, although the levels did not significantly differ among the groups. There were no significant difference in serum AST levels among the groups, but serum ALT levels were significantly higher in the FMT1:1 group than the Control group (P=0.038) (Table 5).

Full table
Hepatic mRNA expression
To elucidate the molecular mechanisms of the effect of FMT on NASH, we evaluated key molecular markers of fibrosis, inflammation, oxidative stress, and cholesterol or lipid metabolism in the liver. The mRNA levels of COL1A1 and α-SMA, which are both responsible for fibrogenesis, tended to be lower in the FMT1:2 group and higher in the FMT1:5 group than the HFC group, although differences were not statistically significant. The mRNA levels of IL-1β, IL-6 and NF-κB, which are involved in inflammation, also tended to be lower in the FMT1:2 group and higher in the FMT1:5 group than the HFC group, although differences were not statistically significant. Also, the mRNA level of HO-1, which is a marker of oxidative stress, tended to be lower in the FMT1:2 group than the HFC group, although difference was not statistically significant. The mRNA level of MnSOD, which is a member of the iron/manganese superoxide dismutase family, tended to be lower in the FMT1:2 and FMT1:5 groups than the HFC group, although differences were not statistically significant. The mRNA level of MTP, which is a rate-limiting protein in the synthesis and excretion of very-low-density lipoprotein from the liver, tended to be higher in the FMT1:5 than the HFC group, although difference was not statistically significant (Figure 2).
Discussion
Established treatments for NAFLD/NASH are few, thus it is vital to develop and validate novel dietary strategies to prevent or attenuate the development of hepatic steatosis and its progression to NASH (6). In the present study, HFC diet supplemented with FMT at a ratio of 1:5 dramatically reduced NASH progression (14%) diagnosed according to the NAS (18) compared to HFC diet without FMT (100%). Serum glucose levels did not significantly differ among the groups, but serum insulin levels tended to be lower in the FMT groups in a green tea extract dose-dependent manner compared to the HFC group, suggesting that green tea leaves reduce insulin resistance. Serum leptin levels also tended to be lower in the FMT groups in a green tea extract dose-dependent manner compared to the HFC group. Insulin resistance is an early event leading to NASH (7), and a high level of serum leptin may contribute to hepatic inflammation and fibrosis in NAFLD (20). Therefore, FMT at a ratio of 1:5 may have a preventive effect on NASH progression.
In the FMT1:5 group, histopathological scores of steatosis were lower compared to the HFC group in the present study. This result was partly due to the synthesis and excretion of very-low-density lipoprotein from the liver, as the mRNA levels of MTP tended to be higher in the FMT1:5 group than the HFC group, although difference was not statistically significant. Also, the mRNA levels of MnSOD, an oxidative stress marker, tended to be lower in the FMT1:5 group than the HFC group, although difference was not statistically significant. However, mRNA levels of proinflammatory genes including MCP-1, IL-1β, IL-6 and NF-κB, and fibrogenetic gene α-SMA tended to be higher in the FMT1:5 group than the HFC group. These results were inconsistent with the lower histopathological scores of lobular inflammation.
On the other hand, HFC diet supplemented with FMT at ratios of 1:2 and 1:1 did not reveal obvious effects on NASH prevention (67% and 86%, respectively) diagnosed according to the NAS (16) compared to the HFC fed rats (100%) in the present study. However, the mRNA levels of proinflammatory genes including TNF-α, IL-1β, IL-6 and NF-κB, and the lipid metabolism-related genes FAS and AOX tended to be lower in the FMT1:2 group than the HFC group. Moreover, mRNA levels of the fibrogenic genes COL1A1 and α-SMA, and the oxidative stress marker genes HO-1 and MnSOD also tended to be lower in the FMT1:2 group than the HFC group. These results may suggest that FMT at a ratio of 1:2 may also have a potential preventive effect on NASH progression.
There were several limitations in the present study. First, the study lacked a group fed HFC diet supplemented exclusively with green tea extract. Because the NAS, serum insulin and leptin levels tended to be lower in the FMT groups in a green tea extract dose-dependent manner compared to the HFC group, green tea leaves may possess a preventive effect on NASH progression. However, our preliminary study showed that HFC diet supplemented with FMT at a ratio of 1:9 had no effect on NASH prevention (unpublished data). Therefore, FMT at a ratio around 1:5 or 1:2 may be a better compounding ratio for the production of black tea polyphenols such as theaflavins and theasinensins in addition to catechins, as reported by Tamaru et al. (15). Second, most of the differences observed in the present study were not statistically significant. Especially, FMT appeared to have no effect on serum AST and ALT concentrations, which are the commonly used marker for hepatocellular injury including NASH (21). The reason is uncertain, but may be due to the no effect on the histopathological lobular inflammation score between HFC and FMT groups, or hepatotoxic effect of FMT. Third, our data suggest that human equivalent dose would be 20–25 g of FMT, which is a large quantity of tea consumption (more than 10 cups of tea per day). The optimal compound (dose) amount of FMT or dosing period should be investigated in a future study.
Conclusions
In the present study, FMT at a ratio of 1:5 or 1:2 likely possessed a preventive effect on NASH progression. FMT at a ratio of 1:5 reduced hepatic steatosis due to the activation of MTP, and FMT at a ratio of 1:2 reduced mRNA levels of some proinflammatory, lipid metabolism-related, fibrogenic and oxidative stress marker genes, suggested that FMT had a preventive effect on NASH progression. In light of the conflicting results between the histopathological and gene expression analyses, further studies are needed to fully elucidate the responsible mechanism(s).
Acknowledgements
This work was supported by a research and development: science and technology research promotion program for agriculture, forestry, fisheries and food industry (research and development projects for application in promoting new policy of agriculture, forestry and fisheries).
Footnote
Conflict of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: All procedures performed on the animals were approved by the Animal Use Committee of the University of Nagasaki (No. 27-14), and the animals were maintained in accordance with the Guidelines for the Care and Use of Laboratory Animals, University of Nagasaki.
References
- Calzadilla Bertot L, Adams LA. The Natural Course of Non-Alcoholic Fatty Liver Disease. Int J Mol Sci 2016;17:E774. [Crossref] [PubMed]
- Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology 1998;114:842-5. [Crossref] [PubMed]
- Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology 2010;52:1836-46. [Crossref] [PubMed]
- Perazzo H, Dufour JF. The therapeutic landscape of non-alcoholic steatohepatitis. Liver Int 2017;37:634-47. [Crossref]
- Tanaka K, Tamaru S, Nishizono S, et al. Hypotriacylglycerolemic and antiobesity properties of a new fermented tea product obtained by tea-rolling processing of third-crop green tea (Camellia sinensis) leaves and loquat (Eriobotrya japonica) leaves. Biosci Biotechnol Biochem 2010;74:1606-12. [Crossref] [PubMed]
- Masterjohn C, Bruno RS. Therapeutic potential of green tea in nonalcoholic fatty liver disease. Nutr Rev 2012;70:41-56. [Crossref] [PubMed]
- Li J, Sapper TN, Mah E, et al. Green tea extract provides extensive Nrf2-independent protection against lipid accumulation and NFκB pro- inflammatory responses during nonalcoholic steatohepatitis in mice fed a high-fat diet. Mol Nutr Food Res 2016;60:858-70. [Crossref] [PubMed]
- Hirsch N, Konstantinov A, Anavi S, et al. Prolonged feeding with green tea polyphenols exacerbates cholesterol-induced fatty liver disease in mice. Mol Nutr Food Res 2016;60:2542-53. [Crossref] [PubMed]
- Teschke R, Zhang L, Melzer L, et al. Green tea extract and the risk of drug-induced liver injury. Expert Opin Drug Metab Toxicol 2014;10:1663-76. [Crossref] [PubMed]
- Teschke R, Larrey D, Melchart D, et al. Traditional chinese medicine (TCM) and herbal hepatotoxicity: RUCAM and the role of novel diagnostic biomarkers such as MicroRNAs. Medicines (Basel) 2016;3:18-78. [PubMed]
- Du YT, Wang X, Wu XD, et al. Keemun black tea extract contains potent fatty acid synthase inhibitors and reduces food intake and body weight of rats via oral administration. J Enzyme Inhib Med Chem 2005;20:349-56. [Crossref] [PubMed]
- Cai X, Fang C, Hayashi S, et al. Pu-erh tea extract ameliorates high-fat diet-induced nonalcoholic steatohepatitis and insulin resistance by modulating hepatic IL-6/STAT3 signaling in mice. J Gastroenterol 2016;51:819-29. [Crossref] [PubMed]
- Miyata Y, Kubayashi T, Tajima K, et al. Development and Functionality of a Mixed Fermented Tea, Goto Tsubaki-cha, Obtained by Tea-rolling Processing of Camellia (Camellia japonica) and Green Tea Leaves. Nippon Shokuhin Kagaku Kogaku Kaisi 2015;62:123-9. [Crossref]
- Miyata Y, Tanaka T, Tamaya K, et al. Technological Development of a Simple and Rapid Method of Producing Fermented Teas Using Different Kinds of Tea Leaves, and the Manufacturing of New Types of Mixed Fermented Teas. Nippon Shokuhin Kagaku Kogaku Kaisi 2011;58:403-12. [Crossref]
- Tamaru S, Ohmachi K, Miyata Y, et al. Hypotriglyceridemic potential of fermented mixed tea made with third-crop green tea leaves and camellia (Camellia japonica) leaves in Sprague-Dawley rats. J Agric Food Chem 2013;61:5817-23. [Crossref] [PubMed]
- Ichimura M, Kawase M, Masuzumi M, et al. High-fat and high-cholesterol diet rapidly induces non-alcoholic steatohepatitis with advanced fibrosis in Sprague-Dawley rats. Hepatol Res 2015;45:458-69. [Crossref] [PubMed]
- Ichimura M, Masuzumi M, Kawase M, et al. A diet-induced Sprague-Dawley rat model of nonalcoholic steatohepatitis-related cirrhosis. J Nutr Biochem 2017;40:62-69. [Crossref] [PubMed]
- Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313-21. [Crossref] [PubMed]
- Matteoni CA, Younossi ZM, Gramlich T, et al. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology 1999;116:1413-9. [Crossref] [PubMed]
- Polyzos SA, Aronis KN, Kountouras J, et al. Circulating leptin in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Diabetologia 2016;59:30-43. [Crossref] [PubMed]
- Kunde SS, Lazenby AJ, Clements RH, et al. Spectrum of NAFLD and diagnostic implications of the proposed new normal range for serum ALT in obese women. Hepatology 2005;42:650-6. [Crossref] [PubMed]


