The day will come to treat HCC in a drugstore?
Hepatocellular carcinoma (HCC) is a refractory cancer and the second-leading cause of death worldwide. HCC is characterized by multicentric development, which accounts for unexpected recurrences even after successful treatment has been performed for the primary tumor, particularly the background liver exerts chronic inflammation and/or advanced fibrosis. The standard treatment for HCC includes partial hepatectomy, radiofrequency ablation, and transcatheter chemoembolization. However, when liver tumors progress to the advanced stages, there are few therapeutic approaches available. Although sorafenib is the only molecular-targeted agent available for advanced HCC, the treatment outcomes are not satisfactory. As such, more therapeutic options are necessary to control HCC. In addition, no effective strategies for HCC prevention have yet been developed, even for patients with risk factors for HCC.
The gut-liver axis has much attention in the development of liver diseases, including nonalcoholic steatohepatitis (NASH), liver cirrhosis, and HCC. Because the composition of the gut microbiota is altered in many liver diseases, restructuring the gut microbiota is an emerging target for therapy. Meta-analysis studies have shown that probiotics treatment modifies the composition of gut microbiota and provides a beneficial effect for patients with NASH (1). In experimental models, gut sterilization by antibiotics treatment reduced the development of HCC in mice (2). Accumulating evidence indicates that these effects of the gut microbiota are mediated by innate and acquired immune systems. For instance, advanced liver diseases allow for the translocation of bacterial components from the gut (3), which is caused by overgrowth of intestinal bacteria, dysbiosis, and increased permeability of the gut. Bacterial components are ligands for Toll-like receptors (TLRs) and activate the innate immune system, resulting in the development of inflammation. In addition, the gut microbiota can regulate T cells that also mediate the acquired immune system. For instance, certain Clostridium and Bacteroidetes species can increase the populations of intestinal Treg cells (4), which produce the anti-inflammatory cytokine IL-10. In contrast, segmented filamentous bacteria (SFB) can induce differentiation of Th17 cells (5), which produce the proinflammatory cytokine IL-17. These T cells maintain the immune system in the gut as well as the whole body. Of note, these gut-derived T cells can migrate to an inflamed microenvironment, in which the T cells are likely to interact with tumor cells as well as immune cells.
Li et al. uncovered a molecular mechanism by which probiotics suppress HCC in mice (6). Their study strongly attracted our interests for the following reasons: (I) probiotics inhibited the expansion of an extraintestinal tumor; (II) pretreatment with probiotics was more effective in suppressing the tumor; (III) probiotics regulated T cell polarization and distribution in the tumor; (IV) IL-17 mediated angiogenesis for tumor growth. Figure 1 summarizes the findings of that study (Figure 1).
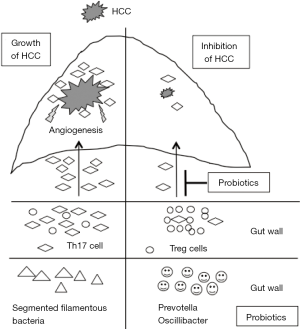
Probiotics are well documented to suppress intestinal inflammation, but their effects of probiotics on extraintestinal organs are largely unknown. Recent studies have shown that the gut microbiota is associated with the development of respiratory diseases and autoimmune diseases, suggesting that probiotics may influence extraintestinal organs. Among the extraintestinal organs, the liver is the most viable target because it receives a larger amount of substances from the gut via the portal vein. Li et al. demonstrated for the first time that probiotics suppressed the growth of HCC, particular when administrated before the inoculation of tumor cells in mice. They clearly showed that T cells mediated tumor suppression by restructuring the gut microbiota composition. Although probiotics treatment was expected to remodel the composition of gut microbiota, this treatment further increased the population of certain beneficial bacteria, including Prevotella and Oscillibacter, which can generate anti-inflammatory fatty acids such as propionate and docosahexaenoate. In addition, these bacteria stimulate immune cells to produce anti-inflammatory cytokines IL-10. In contrast, probiotics reduced the abundance of SFB, an inducer of IL-17. These changes resulted in an increased abundance of anti-inflammatory T cells but a decreased abundance of Th17 cells in the small intestine as well as peripheral blood. As a result, the number of Th17 cells that migrate into the inflamed site was decreased by probiotics treatment. In addition, probiotics reduced the chemotaxis of Th17 cells through the CCL20/CCR6 axis. The probiotics decreased the expression of chemokine receptor CCR6 that recruits Th17 cells. Because HCC can produce a chemokine CCL20 that recruits CCR6-expressing cells (7), probiotics largely blocked the migration of Th17 cells. Furthermore, pretreatment with probiotics effectively suppressed tumor growth. Early exposure to probiotics may educate T cells to limit their differentiation to Th17 cells, which shifts the anti-inflammatory state in the whole body. These data suggest that probiotics have the potential to prevent extraintestinal cancers, including HCC, in which the background liver is affected by chronic inflammation.
Angiogenesis is a key factor in the development of HCC. As proven by sorafenib, angiogenesis is a target for HCC treatment. In addition, inhibitors of VEGF receptors are currently under clinical trials to determine their efficacy in treating HCC (8). Although most previous studies have focused on the VEGF signaling to block angiogenesis, Li et al. highlighted IL-17-dependent angiogenesis. Because IL-17 functions as a chemokine that recruits inflammatory cells, other mediators activated by IL-17 also participate in angiogenesis. Indeed, IL-17 increased the expression of chemokine that recruit inflammatory cells in the liver (9). The IL-17 levels were increased in the small intestine as well as in the portal vein in an experimental NASH model (9), indicating that the liver is exposed to angiogenetic factors from the gut. Given the low abundance of Th17 cells in the healthy liver, IL-17 produced from the intestine may contribute to the development of HCC. We were therefore surprised to learn that an anti-IL-17 antibody drastically suppressed tumor growth compared with probiotics treatment. These data showed the potential utility of IL-17 inhibitors as anti-angiogenetic agents in HCC therapy. Th17/Treg imbalance is observed in patients with HCV (10) and HBV infections (11), with Th17 dominant to Treg. Thus, IL-17 is a potential target for HCC treatment in humans. IL-17 inhibitors are currently used for the treatment of psoriatic arthritis and have shown favorable results (12).
Although Li et al. illustrated that probiotics have the potential to suppress HCC development as well as background inflammation, we should clarify further molecular mechanisms if probiotics are considered as agents for HCC treatment. The authors investigated the effects of probiotics using a well-established model, but HCC cells were transplanted subcutaneously, not in the liver. Because immune cells may induce different reactions between the skin and the liver, it is necessary to test the probiotics in an orthotopic model, as the authors are planning. Gomes et al. recently reported that IL-17 promotes HCC by inducing insulin resistance in the adipose tissue (13), suggesting that additional mechanisms are involved in tumor suppression. However, the role of Th17 is not so simple. For instance, Th17 cells maintain metabolic homeostasis in the gut. Hong et al. reported that the number of Th17 cells was decreased in the gut of mice on a high-fat diet (14). The transfer of Th17 cells to obese mice restored the metabolic disturbance, indicating that Th17 cells play a beneficial role in the host. In addition, clinical studies have shown that an anti-IL-17 antibody failed to treat inflammatory bowel diseases under conditions of increased number of Th17 cells in the intestine (15). These data raise the question of whether or not Th17 cells are beneficial for the treatment of obesity-related HCC.
One of the limitations of experimental research is that the effects of probiotics are often institution-dependent. This type of research is generally performed at a single animal facility, which may have separate and distinct types of gut microbiota. As a result, phenotype of mice was not reproduced in other institutions. Thus, the effects of the probiotics should be tested in a range of institutions. Whether or not the probiotics exert beneficial effects on HCC even in different environments is of interest, as the composition of gut microbiota differs among countries as well as generations, even in the same habitat.
In conclusion, probiotics exert beneficial effects on the host. Li et al. clearly demonstrated that the gut microbiota is a target for treating extraintestinal cancers via immune system modulation in mice. Probiotics are widely available worldwide. They are inexpensive and safe for long-time use, and people can obtain them from a drugstore. Many readers of this article may be dreaming when HCC can be treated in a drugstore (Figure 2). Further investigations are therefore necessary to make this dream come true.
Acknowledgements
The author thanks Yoko Morita for excellent illustrations.
Funding: This work was supported by KAKENHI (16K09337).
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Ma YY, Li L, Yu CH, et al. Effects of probiotics on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol 2013;19:6911-8. [Crossref] [PubMed]
- Dapito DH, Mencin A, Gwak GY, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell 2012;21:504-16. [Crossref] [PubMed]
- Miura K, Ohnishi H. Role of gut microbiota and Toll-like receptors in nonalcoholic fatty liver disease. World J Gastroenterol 2014;20:7381-91. [Crossref] [PubMed]
- Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013;341:569-73. [Crossref] [PubMed]
- Goto Y, Panea C, Nakato G, et al. Segmented filamentous bacteria antigens presented by intestinal dendritic cells drive mucosal Th17 cell differentiation. Immunity 2014;40:594-607. [Crossref] [PubMed]
- Li J, Sung CY, Lee N, et al. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc Natl Acad Sci U S A 2016;113:E1306-15. [Crossref] [PubMed]
- Chen KJ, Lin SZ, Zhou L, et al. Selective recruitment of regulatory T cell through CCR6-CCL20 in hepatocellular carcinoma fosters tumor progression and predicts poor prognosis. PLoS One 2011;6:e24671. [Crossref] [PubMed]
- Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389:56-66. [Crossref] [PubMed]
- Ishioka M, Miura K, Minami S, et al. Altered Gut Microbiota Composition and Immune Response in Experimental Steatohepatitis Mouse Models. Dig Dis Sci 2017;62:396-406. [Crossref] [PubMed]
- Hao C, Zhou Y, He Y, et al. Imbalance of regulatory T cells and T helper type 17 cells in patients with chronic hepatitis C. Immunology 2014;143:531-8. [Crossref] [PubMed]
- Li K, Liu H, Guo T. Th17/Treg imbalance is an indicator of liver cirrhosis process and a risk factor for HCC occurrence in HBV patients. Clin Res Hepatol Gastroenterol 2017;41:399-407. [Crossref] [PubMed]
- McInnes IB, Mease PJ, Kirkham B, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2015;386:1137-46. [Crossref] [PubMed]
- Gomes AL, Teijeiro A, Burén S, et al. Metabolic Inflammation-Associated IL-17A Causes Non-alcoholic Steatohepatitis and Hepatocellular Carcinoma. Cancer Cell 2016;30:161-75. [Crossref] [PubMed]
- Hong CP, Park A, Yang BG, et al. Gut-Specific Delivery of T-Helper 17 Cells Reduces Obesity and Insulin Resistance in Mice. Gastroenterology 2017;152:1998-2010. [Crossref] [PubMed]
- Targan SR, Feagan B, Vermeire S, et al. A Randomized, Double-Blind, Placebo-Controlled Phase 2 Study of Brodalumab in Patients With Moderate-to-Severe Crohn's Disease. Am J Gastroenterol 2016;111:1599-607. [Crossref] [PubMed]