The impact of perioperative blood transfusions on short-term outcomes following hepatectomy
Introduction
Hepatectomy is increasingly performed for a range of malignant and benign diseases. With improvements in perioperative management and surgical technique, post-hepatectomy short-term outcomes have improved and mortality now nears 1% in high volume centres (1,2). However, blood loss remains a significant concern and has been the topic of numerous studies (3-5). As a result, red blood cell transfusions (RBCT) are required in up to 25% of patients undergoing liver resection (6-8). Thus, hepatectomy represents a unique challenge with regards to blood management strategies.
In addition to traditional well-known risks of haemolytic reaction and infection transmission, RBCTs have also been associated with transfusion-related immunomodulation (9,10). By reducing the immunoresponsiveness of the recipient, RBCTs may create a fertile ground for infections or even tumor growth, which may negatively impact the post-operative course (10,11). Increased post-operative morbidity and mortality, as well as delayed recovery, have been reported (12-14).
Recently, both the American Medical Association and the Choosing Wisely Campaign have highlighted RBCT as an overused treatment to be targeted in quality improvement initiatives (15,16). Indeed, transfusion practices vary significantly in both overall rates of RBCT use and triggers for RBCT administration (7,17). A number of medical societies have endorsed the use of restrictive transfusion strategies, based on evidence from randomized controlled trials (18-23). In order to improve adherence to those recommendations and reduce variation in transfusion practice for hepatectomy patients, evidence regarding the impact of RBCT on outcomes is needed. Since hepatectomy presents a very different baseline risk for blood loss and RBCT and a unique morbidity profile, efforts to improve perioperative blood management must be tailored to the specificities of the procedure. Current data regarding the effects of RBCT on post-hepatectomy outcomes either relies on small sample sizes and single institution studies, or is diluted with other type of surgical procedures from which data cannot be extrapolated (6,24,25).
This study employs the large multi-institutional registry of the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) to examine the association between perioperative RBCTs and short-term post-operative outcomes following hepatectomy in order to provide the data to support improvement in transfusion practices as it relates to hepatectomy.
Methods
Study design
A retrospective cohort study of patients undergoing elective hepatectomy was conducted, with the exposure of interest being perioperative RBCT. Short-term post-operative outcomes were compared between patients who received RBCT and those who did not.
Approval was sought through the Sunnybrook Health Sciences Centre Research Ethics Board and was not deemed necessary by the board. The study was conducted and reported according to the recommendations of the RECORD statement (26).
Data sources
We used data from the ACS-NSQIP registry. The ACS-NSQIP is a multicenter prospective registry designed for quality improvement by providing participating centres with risk-adjusted outcomes for surgical patients. At the end of 2014, the ACS-NSQIP included more than 525 hospitals from academic and community settings, representative of various regions in North America. Over 275 variables are collected, including demographics, pre-operative risk factors, procedural indication and details, and 30-day post-operative morbidity and mortality. Data are collected by trained data abstractors and audited for accuracy (27). The methods of ACS-NSQIP abstractors training, data collection process, and reliability audits have previously been reported (27-30).
Study cohort
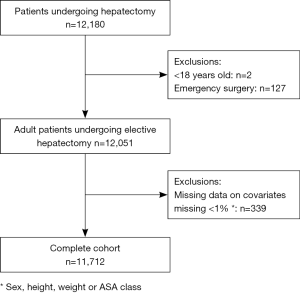
Using the ACS-NSQIP Participant User File (PUF), patients from participating institutions entered in the registry between January 1, 2007 and December 31, 2012, who underwent a hepatectomy [Current Procedural Terminology (CPT) codes 47120, 47122, 47125, and 47130] were considered eligible for the study. Exclusion criteria included an emergent operation, age <18 years old, and missing demographic data (gender, height, weight or ASA class). Less than 1% of the cohort was excluded for missing data on these covariates.
Exposure
The exposure was perioperative RBCT, defined as the receipt of RBCT intra-operatively or in the 72 hours post-operatively (1). Coding of blood transfusion in ACS-NSQIP changed over the study interval—any transfusion was recorded as a dichotomous (yes or no) variable prior to 2010, and the number (RBC units) of transfusions received was captured as an ordinal variable from 2010 onwards. Therefore, a dichotomous transfusion variable was created for any transfusion of one or more RBC units for 2010 to 2012. RBCT provided prior to surgery were not included.
Outcomes
The primary outcomes were 30-day major morbidity and mortality. A major morbidity composite outcome was created based on the occurrence of at least one of the following: deep or organ-space surgical site infection (SSI), wound dehiscence, pneumonia, pulmonary embolism, prolonged mechanical ventilation beyond 48 hours, unplanned re-intubation, renal failure, sepsis, myocardial infarction, cardiac arrest, or cerebrovascular accident (31). Post-operative mortality was defined as death within 30 days of operation.
Secondary outcomes included system-specific 30-day morbidity grouped into: (I) post-operative infections (superficial, deep and organ space-space SSI, pneumonia, urinary tract infection, sepsis, septic shock); (II) cardiac events (myocardial infarction, cardiac arrest); (III) respiratory failure (prolonged mechanical ventilation beyond 48 hours, unplanned re-intubation); and (IV) venous thrombo-embolic events (pulmonary embolism, deep vein thrombosis), as well as unplanned re-operation, and hospital length of stay (LOS) (1).
Covariates
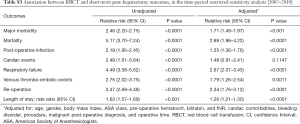
Data on patients’ baseline demographics (age, gender, race), clinical characteristics [pre-operative comorbidities, body mass index (BMI), American Society of Anesthesiologists (ASA) score, and biochemical values including albumin, haemoglobin, bilirubin, and INR], diagnosis (malignant vs. benign based on ICD-9 codes 150 to 199.x, 209.x, 236.x, 237.x, and 238.x), and treatment-related details (surgical procedure, operative time, year of surgery) were abstracted from the ACS-NSQIP registry (32). Cardiac co-morbidities were defined as history of congestive heart failure in the 30 days prior to surgery, myocardial infarction in the 6 months prior to surgery, angina in the 30 days prior to surgery, or medicated hypertension within 30 days prior to surgery. Surgical procedures were grouped into partial lobectomy (CPT 47120), lobectomy (CPT 47125 and 47130), and trisegmentectomy (CPT 47122).
We identified a priori highly relevant patient and operative characteristics as potential confounders of the relationship between RBCT and outcomes. Covariates thus were selected based on timing (known pre-operatively), clinical relevance (considered when assessing a patient for risk of adverse perioperative events or of receiving RBCT) and existing literature (established relationship with worse surgical outcomes). The most parsimonious set of covariates was selected to maintain adequate study power. The following covariates were ultimately included: age, gender, BMI, ASA class, pre-operative hematocrit, INR, and bilirubin, cardiac comorbidities, bleeding disorder, malignant diagnosis, the surgical procedure, and operative time.
Statistical analysis
Descriptive analysis was first performed to assess the characteristics of transfused patients and compare them to those not transfused. Unknown categories were created for missing data. Categorical data were reported as absolute number (n) and proportion (%), and continuous data as mean or median with interquartile range (IQR). Baseline patient variables and operative characteristics were compared between patients who received a transfusion and those who did not. Chi square tests for independence were used to compare categorical variables. Normally distributed continuous data were compared using t-tests and skewed continuous data using Wilcoxon-rank sum tests.
Modified Poisson regression analysis was used to examine the association between RBCT for common dichotomous outcomes (>10%), and logistic regression for uncommon dichotomous outcomes (≤10%). These regression methods allow for the estimation and interpretation of relative risks (RR) with 95% confidence intervals (95% CI). LOS values were treated as count data but the data was skewed and violated the assumptions of Poisson regression, therefore negative binomial regression was used to study the relationship between transfusion and LOS (33). Similar to Poisson regression, negative binomial regression allows for the estimation of interpretation of incidence rate ratios with 95%CI. Incidence rate ratios are a measure of the number of events (here number of days hospitalized) per time (here all patients were followed for 30 days). Multivariate analyses were adjusted for previously described covariates defined a priori.
Finally, sensitivity analyses were conducted to assess the robustness of the results. Because operative time may be considered on the causal pathway for the relationship between RBCT and major morbidity, we constructed multivariate models with and without it as a covariate. We also explored the impact of missing data on the results. First, we conducted a case-deletion analysis by excluding patients with missing data on the key covariates in the relationship between RBCT and morbidity (pre-operative hematocrit and cardiac comorbidities), if missing in ≥1% of the cohort. Second, since important comorbidity data were missing in specific calendar years, we restricted the analysis to patients who were operated during the timeframe with complete data [2007–2010] (Figure S1, Tables S1-S3).

Full table
Full table
Full table
All statistical analyses were conducted using SAS 9.3 for Windows (SAS Institute Inc., Cary, USA). P values of less than 0.05 were considered statistically significant. ALM and JH had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Results
A total of 12,180 patients undergoing hepatectomy were identified in the ACS-NSQIP registry over the study period. After applying exclusion criteria, 11,712 patients were included in the complete case cohort for this study (Figure 1). Of those, 2,940 (25.1%) received RBCT.
Baseline characteristics of the included patients are presented in Table 1. Patients receiving RBCT were more commonly of older age, advanced ASA score (III or IV/V), and higher BMI than those not receiving RBCT. Transfused patients presented a heavier burden of pre-operative comorbidities than those not transfused, including higher prevalence of diabetes, dyspnea, cardiovascular disease, dialysis, bleeding disorders, ascites, and weight loss. Among patients operated for benign disease, 22.9% received RBCT. The extent of surgery differed significantly between transfused and non-transfused patients (P<0.001). Trisegmental lobectomy (14.3% vs. 8.1%) and lobectomy (38.2% vs. 27.0%) were more common with RBCT, whereas partial lobectomy (47.5% vs. 64.9%) was more common for non-transfused patients. Median operative time was longer in the RBCT group with 309.2 (SD: 173) minutes compared to 221 (SD: 124) minutes for the no RBCT group (P<0.01).
Full table
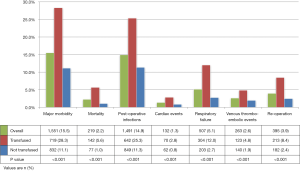
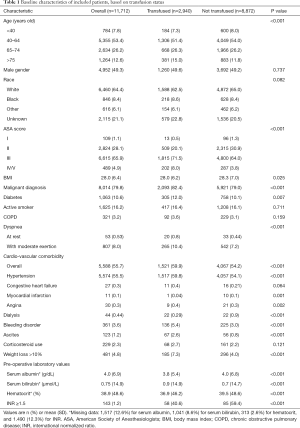
Thirty-day post-hepatectomy morbidity and mortality are depicted in Figure 2. Overall major morbidity (28.3% vs. 11.1%) and mortality (5.6% vs. 1.0%) rates were higher in transfused than non-transfused patients (both P<0.001). The difference in morbidity persisted when classified into system-specific categories, including more frequent post-operative infectious, cardiac, respiratory failure, and venous thrombo-embolic events. Median LOS was longer in the RBCT group, with 10.9 (IQR: 6.6–12.0) days compared to 6.8 (IQR: 3.4–7.0) days (P<0.001).
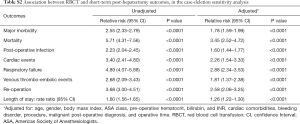
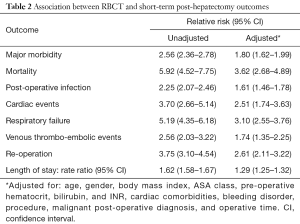
Results of the multivariable analyses are detailed in Table 2. After adjusting for relevant pre- and intra-operative variables, RBCT was independently associated with increased risk of 30-day major morbidity (RR 1.80, 95% CI: 1.62–1.99), and increased risk of 30-day mortality (RR 3.62; 95% CI: 2.68–4.89). The relationship between transfusion and worse peri-operative morbidity held true when looking specifically at post-operative infections, cardiac events, respiratory failure, and venous thrombo-embolic events. Transfused patients had a rate of hospital stay 1.29 times greater than patients who did not receive a transfusion. The mean post-hepatectomy LOS increases from 6.8 days without RBCT to 8.7 days with RBCT, when adjusting for relevant covariates, with a rate of hospital stay 1.29 times greater for transfused than not transfused patients.
Full table
Sensitivity analyses were conducted to explore the inclusion of operative time as a potential confounder as it might also be considered in the causal pathway between RBCT and adverse outcomes. Models with and without this variable had no impact on the conclusions. As the results did not change significantly operative time was controlled for in the final multivariable analyses (Figure S1, Tables S1-S3).
Multivariable analyses were also repeated with two additional ways of handling missing data. When looking at key covariates in assessing the association between RBCT and short-term outcomes, missing data was encountered in no patient for age, 0.1% for ASA class, 0.1% for gender, and 2.6% for pre-operative hematocrit. Data on cardiac comorbidities posted the greatest challenge, with 27% of records lacking these data. Cardiac comorbidity data were missing in selected calendar years: no data were missing on cardiac comorbidities for 2007 to 2010, while 46.0% were missing for this variable in 2011 and 71.4% in 2012. Therefore, we first excluded patients with missing data on key covariates. Second, given the association between missingness of data and year of operation, we restricted the analysis to the time-period with complete data. The impact of RBCT on 30-day major morbidity and mortality was not significantly altered in either analysis (Figure S1, Tables S1-S3). The results consistently indicated an independent relationship between RBCT and adverse outcomes.
Discussion
This study took advantage of the large multi-institutional clinical dataset of the ACS-NSQIP to assess the relationship between RBCT and short-term outcomes following hepatectomy. After adjusting for relevant demographic, clinical, and operative characteristics, major morbidity was independently associated with a near 1.8-fold increase in 30-day major morbidity and 3.6-fold increase in mortality. This independent association was consistent across all sub-categories of major morbidity: post-operative infectious, cardiac, respiratory failure, and venous-thrombo-embolic events. Transfused patients stayed in hospital longer than patients who were not transfused, with a 29% increase in rate of LOS. These results allow surgeons to appreciate the worse outcomes that may be expected with RBCT specifically for hepatectomy.
Transfusion-related immunomodulation is hypothesized to explain the detrimental impact of RBCT on post-operative outcomes (10,11). Immunomodulation is thought to be mediated by a variety of mechanisms, including induction of suppressor T cells, suppression of natural killer antibodies, induction of apoptosis, accumulation of factors inhibiting neutrophils activity, and increased levels of soluble HLA class I peptides (10). The clinical effects of transfusion-related immunomodulation were first observed in allogeneic kidney transplant and Crohn’s disease patients who experienced respectively longer graft survival and fewer recurrences when receiving RBCT (34-36).
Worse morbidity and mortality with RBCT following gastrointestinal surgery have previously been highlighted (25,37). These analyses mixed different procedures with variable baseline risks for transfusion and morbidity profiles. Hepatectomy presents a unique risk for blood loss and transfusions, mostly due to the intrinsic nature of the liver parenchyma. As such it requires specific intra- and post-operative management strategies not commonly utilized for other surgical procedures and may influence post-operative outcomes (3-5). Evidence of RBCT impact on morbidity pertaining specifically to liver resection remains limited. All studies rely on retrospective data from single centres (24,38,39). The largest such study reported increased morbidity, mortality, and LOS in transfused patients, but dates back to the 1980s and 1990s (24). Two other studies used contemporary data and revealed similar associations between RBCT and morbidity, but were of limited sample size (38,39). The current study represents a unique addition to the literature as it takes into account the uniqueness of liver resection, uses contemporary data, and is the first multi-institutional appraisal of the relationship between RBCT and short-term post-operative outcomes.
Despite randomized controlled trials and associated clinical practice guidelines supporting restrictive transfusion strategies in critical care and surgical settings, transfusion practices vary significantly (7,17-23). As many as one out of two RBCT administered in hepatobiliary surgery is considered unnecessary according to those guidelines (7,18,40). While some RBCT are clinically necessary for patient care, a significant proportion could be avoided thus limiting patients’ exposure to unnecessary risk of threatening their post-operative recovery.
Comprehensive blood management programs can effectively reduce the use of perioperative RBCT as well as improve post-operative outcomes and save costs (41-44). However, the successful implementation of these programs requires buy-in from primary stakeholders caring for the patients. Procedure-specific data that directly pertain to one’s practice is necessary to ensure uptake of such quality improvement initiatives. Indeed, tailored interventions driven by data directly applicable to a specific practice are known to be critical in ensuring successful and lasting uptake of clinical practice guidelines (45,46). By taking advantage of the large multi-institutional ACS-NSQIP dataset, this study focused on hepatectomies to provide specific data to support the rationale for and motivate physicians to partake in blood management initiatives for patients undergoing liver resections.
It is acknowledged that this study is limited by biases inherent to retrospective designs. In particular, despite detailed multivariable analyses, unknown confounders and variables cannot be accounted for. The analysis is limited by the information available in the ACS-NSQIP. In particular, it was not possible to identify the exact indication for transfusion since data on pre-transfusion haemoglobin levels, symptoms, or vital signs were not available. Thus, it was not possible to tease out the increased in morbidity related to avoidable versus necessary RBCT.
Nevertheless, the ACS-NSQIP provides multi-institutional clinical data captured by rigorously trained data abstractors and subjected to frequent audits to ensure accuracy, thereby limiting information bias (27-30). With such large datasets, missing data can present an issue and the ACS-NSQIP is not exempt (47-49). Previous studies of ACS-NSQIP indicated that missing data was more common in healthier patients. When looking at how to best handle missing data, no significant change was observed between various strategies to handle missing data; therefore, no particular method could be recommended (48). In this analysis, when looking at key covariates in assessing the association between RBCT and short-term outcomes, missing data on cardiac comorbidities was encountered in 27.3% of records. Data were missing according to the year of operation, which likely relates to the year of data collection, and increased over time. To avoid biasing the effects estimate by excluding patients with missing data, all patients were included in the primary analysis. Our results were robust to a number of methods of handling missing data, which strengthens the validity of the current analysis.
Conclusions
In this study, perioperative RBCT was independently associated with worse 30-day morbidity and mortality following elective hepatectomy. All sub-types of morbidity were impacted, whether infectious, cardiac, respiratory, or thrombo-embolic. LOS was significantly prolonged in transfused patients. This data provides hepatectomy-specific information to further support the need for comprehensive blood management strategies aimed at safely reducing the use of RBCT.
Acknowledgements
None.
Footnote
Conflicts of Interest: This work has been presented as poster presentation at the 2015 Canadian Surgery Forum in Québec City, in September 2015.
Ethical Statement: Approval was sought through the Sunnybrook Health Sciences Centre Research Ethics Board and was not deemed necessary by the board. The study was conducted and reported according to the recommendations of the RECORD statement.
References
- Jarnagin WR, Gonen M, Fong Y, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg 2002;236:397-406. [Crossref] [PubMed]
- Belghiti J, Hiramatsu K, Benoist S, et al. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg 2000;191:38-46. [Crossref] [PubMed]
- Gurusamy KS, Li J, Vaughan J, et al. Cardiopulmonary interventions to decrease blood loss and blood transfusion requirements for liver resection. Cochrane Database Syst Rev 2012.CD007338. [PubMed]
- Gurusamy KS, Li J, Sharma D, et al. Pharmacological interventions to decrease blood loss and blood transfusion requirements for liver resection. Cochrane Database Syst Rev 2009.CD008085. [PubMed]
- Cheng ES, Hallet J, Hanna SS, et al. Is central venous pressure still relevant in the contemporary era of liver resection? J Surg Res 2016;200:139-46. [Crossref] [PubMed]
- Lucas DJ, Schexneider KI, Weiss M, et al. Trends and risk factors for transfusion in hepatopancreatobiliary surgery. J Gastrointest Surg 2014;18:719-28. [Crossref] [PubMed]
- Ejaz A, Spolverato G, Kim Y, et al. Identifying Variations in Blood Use Based on Hemoglobin Transfusion Trigger and Target among Hepatopancreaticobiliary Surgeons. J Am Coll Surg 2014;219:217-28. [Crossref] [PubMed]
- Hallet J, Tsang M, Cheng ES, et al. The Impact of Perioperative Red Blood Cell Transfusions on Long-Term Outcomes after Hepatectomy for Colorectal Liver Metastases. Ann Surg Oncol 2015;22:4038-45. [Crossref] [PubMed]
- O’Brien SF, Yi QL, Fan W, et al. Current incidence and residual risk of HIV, HBV and HCV at Canadian Blood Services. Vox Sang 2012;103:83-6. [Crossref] [PubMed]
- Vamvakas EC, Blajchman MA. Transfusion-related immunomodulation (TRIM): an update. Blood Rev 2007;21:327-48. [Crossref] [PubMed]
- Blumberg N, Heal JM. Effects of transfusion on immune function. Cancer recurrence and infection. Arch Pathol Lab Med 1994;118:371-9. [PubMed]
- Acheson AG, Brookes MJ, Spahn DR. Effects of allogeneic red blood cell transfusions on clinical outcomes in patients undergoing colorectal cancer surgery: a systematic review and meta-analysis. Ann Surg 2012;256:235-44. [Crossref] [PubMed]
- Braga M, Vignali A, Radaelli G, et al. Association between perioperative blood transfusion and postoperative infection in patients having elective operations for gastrointestinal cancer. Eur J Surg 1992;158:531-6. [PubMed]
- Amato A, Pescatori M. Perioperative blood transfusions for the recurrence of colorectal cancer. Cochrane Database Syst Rev 2006.CD005033. [PubMed]
- Joint Commission of the American Medical Association. Proceedings from the National Summit on Overuse (Internet). (cited 2014 Nov). Available online: http://www.jointcommission.org/overuse_summit/
- Callum JL, Waters JH, Shaz BH, et al. The AABB recommendations for the Choosing Wiselycampaign of the American Board of Internal Medicine. Transfusion 2014;54:2344-52. [Crossref] [PubMed]
- Hutton B, Fergusson D, Tinmouth A, et al. Transfusion rates vary significantly amongst Canadian medical centres. Can J Anaesth 2005;52:581-90. [Crossref] [PubMed]
- Carson JL, Grossman BJ, Kleinman S, et al. Red blood cell transfusion: a clinical practice guideline from the AABB*. Ann Int Med 2012;157:49-58. [Crossref] [PubMed]
- Practice Guidelines for blood component therapy: A report by the American Society of Anesthesiologists Task Force on Blood Component Therapy. Anesthesiology 1996;84:732-47. [Crossref] [PubMed]
- Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med 1999;340:409-17. [Crossref] [PubMed]
- Carson JL, Terrin ML, Sanders DW, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med 2011;365:2453-62. [Crossref] [PubMed]
- Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med 2013;368:11-21. [Crossref] [PubMed]
- de Almeida JP, Vincent JL, Galas FR, et al. Transfusion requirements in surgical oncology patients: a prospective, randomized controlled trial. Anesthesiology 2015;122:29-38. [Crossref] [PubMed]
- Kooby DA, Stockman J, Ben-Porat L, et al. Influence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases. Ann Surg 2003;237:860-9; discussion 869-70. [Crossref] [PubMed]
- Ferraris VA, Davenport DL, Saha SP, et al. Intraoperative transfusion of small amounts of blood heralds worse postoperative outcome in patients having noncardiac thoracic operations. Ann Thorac Surg 2011;91:1674-80. [Crossref] [PubMed]
- Benchimol EI, Smeeth L, Guttmann A, et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) Statement. PLoS Med 2015;12:e1001885. [Crossref] [PubMed]
- Shiloach M, Frencher SK, Steeger JE, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg 2010;210:6-16. [Crossref] [PubMed]
- Henderson WG, Daley J. Design and statistical methodology of the National Surgical Quality Improvement Program: why is it what it is? Am J Surg 2009;198:S19-27. [Crossref] [PubMed]
- Cohen ME, Ko CY, Bilimoria KY, et al. Optimizing ACS NSQIP modeling for evaluation of surgical quality and risk: patient risk adjustment, procedure mix adjustment, shrinkage adjustment, and surgical focus. J Am Coll Surg 2013;217:336-46.e1. [Crossref] [PubMed]
- Davenport DL, Holsapple CW, Conigliaro J. Assessing surgical quality using administrative and clinical data sets: a direct comparison of the University HealthSystem Consortium Clinical Database and the National Surgical Quality Improvement Program data set. Am J Med Qual 2009;24:395-402. [Crossref] [PubMed]
- Parikh P, Shiloach M, Cohen ME, et al. Pancreatectomy risk calculator: an ACS-NSQIP resource. HPB 2010;12:488-97. [Crossref] [PubMed]
- The World Health Organization. Manual of the internatioanl statistical classification of diseases, injuries, and causes of death (9th revision). Geneva: The World Health Organization, 1976.
- Gardner W, Mulvey EP, Shaw EC. Regression analyses of counts and rates: Poisson, overdispersed Poisson, and negative binomial models. Psychol Bull 1995;118:392-404. [Crossref] [PubMed]
- Opelz G. Improved kidney graft survival in nontransfused recipients. Transplant Proc 1987;19:149-52. [PubMed]
- de la Cruz JS, Sally MB, Zatarain JR, et al. The impact of blood transfusions in deceased organ donors on the outcomes of 1,884 renal grafts from United Network for Organ Sharing Region 5. J Trauma Acute Care Surg 2015;79:S164-70. [Crossref] [PubMed]
- Peters WR, Fry RD, Fleshman JW, et al. Multiple blood transfusions reduce the recurrence rate of Crohn's disease. Dis Colon Rectum 1989;32:749-53. [Crossref] [PubMed]
- Bernard AC, Davenport DL, Chang PK, et al. Intraoperative transfusion of 1 U to 2 U packed red blood cells is associated with increased 30-day mortality, surgical-site infection, pneumonia, and sepsis in general surgery patients. J Am Coll Surg 2009;208:931. [Crossref] [PubMed]
- Cannon RM, Brown RE, Hill CR, et al. Negative effects of transfused blood components after hepatectomy for metastatic colorectal cancer. Am Surg 2013;79:35-9. [PubMed]
- Gruttadauria S, Saint Georges Chaumet M, Pagano D, et al. Impact of blood transfusion on early outcome of liver resection for colorectal hepatic metastases. J Surg Oncol 2011;103:140-7. [Crossref] [PubMed]
- Ross A, Mohammed S, Van Buren G, et al. An assessment of the necessity of transfusion during pancreatoduodenectomy. Surgery 2013;154:504-11. [Crossref] [PubMed]
- Wehry J, Agle S, Philips P, et al. Restrictive blood transfusion protocol in malignant upper gastrointestinal and pancreatic resections patients reduces blood transfusions with no increase in patient morbidity. Am J Surg 2015;210:1197-204; discussion 1204-5. [Crossref] [PubMed]
- Freedman J, Luke K, Escobar M, et al. Experience of a network of transfusion coordinators for blood conservation (Ontario Transfusion Coordinators (ONTraC)). Transfusion 2008;48:237-50. [PubMed]
- Froman JP, Mathiason MA, Kallies KJ, et al. The impact of an integrated transfusion reduction initiative in patients undergoing resection for colorectal cancer. Am J Surg 2012;204:944-50. [Crossref] [PubMed]
- LaPar DJ, Crosby IK, Ailawadi G, et al. Blood product conservation is associated with improved outcomes and reduced costs after cardiac surgery. J Thorac Cardiovasc Surg 2013;145:796-803; discussion 803-4. [Crossref] [PubMed]
- Cabana MD, Rand CS, Powe NR, et al. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA 1999;282:1458-65. [Crossref] [PubMed]
- Davis DA, Taylor-Vaisey A. Translating guidelines into practice. CMAJ 1997;157:408-16. [PubMed]
- Rubin DB. Inference and missing data. Biometrika 1976;63:581-92. [Crossref]
- Parsons HM, Henderson WG, Ziegenfuss JY, et al. Missing data and interpretation of cancer surgery outcomes at the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg 2011;213:379-91. [Crossref] [PubMed]
- Hamilton BH, Ko CY, Richards K, et al. Missing data in the American College of Surgeons National Surgical Quality Improvement Program are not missing at random: implications and potential impact on quality assessments. J Am Coll Surg 2010;210:125-39.e2. [Crossref] [PubMed]