The emerging role for robotics in cholecystectomy: the dawn of a new era?
Introduction
The word robot was introduced into popular culture less than a century ago by Czech writers Josef and Karl Kapek (1). In his play Rossum’s Universal Robots, Karl Kapek created a world in which rapid technological growth ultimately led to the downfall of mankind through machine-made “robota” or manual laborers who ultimately turned against their human masters. Despite fascination with these innovative mechanical devices, significant reservations exist as robots take on a larger role in our industrialized society, particularly in the field of medicine.
When the laparoscopic cholecystectomy (LC) was introduced in the mid-1980s, the operation was met with significant opposition due to concerns for safety. Edward Muhe, credited with the first LC, was criticized by his peers who claimed that “large surgeries required large incisions (2)”. In addition, technical challenges such as visual and spatial limitations from two-dimensional imaging, introduction of a fulcrum effect from trocar use, restricted range of motion of laparoscopic instrumentation, and unstable camera control led to the slow adoption of minimally invasive surgical techniques. Barriers to laparoscopy were quickly overcome, however, when the benefits to patients including decreased pain, lower morbidity, and faster recovery became evident. LC is now the standard of care worldwide.
Ironically, the roots of robotic surgery took place in the early years of laparoscopy as an attempt to counter its limitations. While the first robotic surgical systems were developed in the mid-1980s, the robotic platform did not gain widespread acceptance until the da Vinci® telesurgical robotic system (Intuitive Surgical, Sunnyvale, CA, USA) was FDA-approved in 2000 (1,3). Advantages of robotic-assisted versus conventional laparoscopic surgery include a three-dimensional video platform, more versatile instrumentation with 7 rather than 4 degrees of motion, and better ergonomic design for surgeons (4). While robotics has gained significant traction in the fields of urology and gynecology, the technology has been slow to permeate general surgery particularly for operations in which the standard of care is a minimally invasive approach, such as the LC. Increased cost and longer operative times without improvement in patient outcomes have led to significant criticism against robotic-assisted general surgery.
In this review we will discuss the advantages and disadvantages of robotic surgery as they apply specifically to cholecystectomy. We will address robotic cholecystectomy (RC) either by multiple port (MPRC) or single site (SSRC), economic considerations, implications for surgical training, short and long term outcomes, the effect of indocyanine green cholangiography on safe cholecystectomy, and the role of emerging technology.
Techniques for multi-port and single site robotic cholecystectomy
The da Vinci® Surgical System is not a true, independently functioning robot. Rather, it is a computer-aided system in which the surgeon can control robotic arms tele-surgically from a console some distance away from the patient’s bedside. The da Vinci® system has three main components: a surgeon console with a stereoscopic viewer and master controllers for the flexible EndoWrist® instruments, a patient side-cart with three to four pivoting robotic arms designed to hold a camera and instruments, and a vision cart housing image processors for the 3D, high-definition endoscope (5). Intuitive currently manufactures two versions of their tele-surgical robot, the traditional da Vinci Si® launched in 2009 and da Vinci Xi® which was launched in 2014.
MPRC can be performed using three robotic ports with a single assistant port or four robotic ports. Similar to LC, ports can be placed according to surgeon preference. However, a few general principles should be kept in mind. A minimum of 8 cm (da Vinci Xi®) to 10 cm (da Vinci Si®) distance from the target anatomy should be maintained to allow free range of motion of articulating instruments. In addition, each of the ports should be spaced a similar distance apart from each other to prevent instrument collisions. Though a variety of robotic instruments can be used, minimizing the number of instrument exchanges is more cost effective. By limiting instrument exchanges, case times are reduced as well. At our institution, a majority of the dissection is done with the hook cautery. The cystic duct and artery can be clipped, suture ligated, or stapled. The portal system can also be imaged using indocyanine green and the da Vinci FireFly® technology. 3D visualization enhances object identification and reduces confusion of portal anatomy.
SSRC can be performed with flexible instrumentation that is placed through a specifically designed curved cannula that allows triangulation at the surgical field. These cannulas are docked to the robotic arms to allow the operative surgeon to perform the surgery more naturally using right and left-handed instrumentation as the robotic platform corrects for instrument location. The same 3D visual camera system is utilized with the availability of indocyanine green cholangiography.
Minimally invasive surgery at the crossroads: The case for robotic surgery
Despite the clear benefits of minimally invasive surgery on patient outcomes, many complex abdominal operations are predominantly performed open in the United States. A 2013 review of nationwide data from academic institutions demonstrated that only 52% of colectomies and 28% of ventral hernias are performed laparoscopically (6). Similarly, several national studies done within the past five years demonstrate that less than 50% of liver resections (7), fewer than 30% of distal pancreatectomies (8), and only 12% of gastrectomies (9) were performed laparoscopically.
While the underutilization of laparoscopy is likely multifactorial, many argue that the steep learning curve for advanced laparoscopy is a major barrier. In contrast, many complex laparoscopic tasks such as suturing are more easily mastered when a robotic platform is applied (10,11). Some studies suggest that surgical novices are able to gain proficiency in robotic surgery quickly despite lack of prior laparoscopic experience, and that those with experience are able to perform many laparoscopic tasks more quickly when using the robot (12,13).
The cost of robotic surgery
Despite many of the technical advantages of robotic-assisted surgery, cost is a major prohibitive factor. The da Vinci® Surgical System costs $0.6–2.5 million, depending on upgrades and instruments purchased, and requires an annual service contract which can range between $100,000 to $170,000 annually (14). The additional variable cost of a robotic procedure is estimated to be $1,600 per case, which increases to $3,200 per case when the cost of the robot itself is considered (15). Reimbursement rates for robotic surgery parallel rates for standard laparoscopic procedures, often making it difficult to justify the additional expense.
Procedural costs are extremely difficult to evaluate given variability of charge, cost, and payment data across institutions. An early prospective, case-matched study on MPRC found that hospital costs were approximately $1,600 higher when compared to LC (16). The primary difference was attributable to the amortized cost of the robot and consumables for the system. A more recent retrospective study demonstrates increase in total cost for MPRC ($8,870 MPRC vs. $5,771 LC) and a decrease in revenue (-$848 MPRC vs. $186 LC) (17). However there was a higher percentage of chronic cholecystitis in the MPRC cohort (27.9% MPRC vs. 14.4% LC), which may have contributed to the increased hospital costs.
Alternatively, a recent retrospective study of single-site robotic cholecystectomy (SSRC) demonstrated a decrease in median hospital cost ($1,319 SSRC vs. $1,710 LC) (18). Costs were contained by limiting non-essential instruments from standard trays and avoiding disposable items such as a retrieval bag for the specimen. In addition, the authors demonstrated further cost savings after the first 50 SSRC presumably due to a decrease in operative time. Of note, fixed costs such as initial robot investment were not included in this study.
The numbers game: Our institutional experience evaluating robotic costs
Our unpublished, institutional experience has come full circle in the analysis of cholecystectomy. In our first analysis comparing LC to SSRC, SSRC incurred a greater cost to the hospital (Table 1). While there was no statistically significant difference between conventional laparoscopy and robotics in terms of anesthesia or PACU costs, the operating room costs were significantly greater for robotic cases than for laparoscopic cases. These greater costs are the result of two items applied uniquely to robotic cases: (I) depreciation and indirect costs and (II) disposables costs.
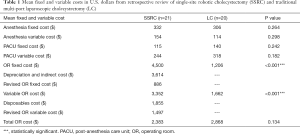
Full table
These additional fixed costs had been arbitrarily assigned to robotic cases as a method to account for the amortized cost of the physical asset as well as the service contract. However, a comparable fixed cost associated with delivering laparoscopic surgery had not been included in this analysis. This cost accounting method thereby affects the perceived cost of cholecystectomy delivered by robotic means.
Between the early and mature phase of our robotic service delivery, the use of robotic instrumentation has been reduced and operating room time has decreased, resulting in an approximately 25–35% decrease in cost associated with SSRC. Analysis of MPRC has also demonstrated a similar reduction in cost.
The contribution of operative time on cost savings is difficult to tease out given the variability of patient presentation. In our experience, patients undergoing elective surgery for biliary colic or chronic cholecystitis both experienced a decrease in operative time on the robotic platform. These results have been seen by other groups with shorter operative times (18,19). The effect of operative time charges must be included in any cost analysis to accurately assess the impact of this technology.
Data based on an early or limited use of robotics has led to inconsistent results and a more critical analysis of robotic procedures. Further study during the mature phase of robotic learning is necessary. Decisions made based on flawed learning curve data may result in an incorrect assessment of value of robotics by both surgeons and administrators responsible for decision making.
Robotic cholecystectomy as a tool for training
Despite the question of cost, use of the RC (both MPRC and SSRC) is considered appropriate in training for more complex abdominal operations (20). As one of the most commonly performed operations in general surgery and one of the few with a standardized approach for maintaining safety, the LC is almost an ideal operation for transitioning into robotic surgery. The first RC was performed by Belgian Jacques Himpens in 1997, marking the first human use of the da Vinci® surgical system (21). Since then, several studies have demonstrated that RC is safe and effective for general surgeons in the early part of their learning curve for robotic surgery (22,23).
The learning curve for RC varies widely, ranging from a mean surgical time of 57–167 minutes (24). Unlike other types of procedures, a robotic procedure involves technical learning of the surgeon, as well as an institutional level learning for operating room staff in setting up and managing robotic equipment. In a study examining this very learning curve, authors found that over the course of 48 RC, the total robotic set-up time (from skin incision to docking completion) decreased from a mean of 30.6 minutes in the first 16 cases to a mean of 18.3 minutes in the last 16 cases (40.2% decrease) (25). The mean robotic operating time (34 minutes) did not change significantly change during the course of the study, presumably due to the institution’s extensive robotic surgery training curriculum and prior experience of the attending surgeon in using the technology for other more complex procedures such as the laparoscopic gastric bypass.
Further analysis of training residents in RC has shown that there is no increase in operative time or increase in complication rates when compared to teaching LC (26,27). These outcomes indicate that RC can be taught to trainees with both consistency and in a time-efficient manner. The reproducibility of these results is a good prognostic indicator that the learning curve for more complex robotic procedures is also reasonable.
Similar evaluation by surgeons moving to SSRC have shown that there is a minimal learning curve and that the outcomes of robotic novices are similar to experts (28). The equivalency in outcomes between those in the early and mature portion of their learning curve suggests the technology does not place patients or hospitals at increased risk with utilization, but may level the playing field in terms of technical expertise in performing laparoscopic procedures. This finding is significantly different from what is seen in non-robotic single-site LC, which has not gained favor with the majority of surgeons due to the complexity of the technique and complications seen (29).
Outcomes of robotic cholecystectomy: Safety and patient satisfaction
Multiple studies have demonstrated that the outcomes of RC are similar to LC with regards to initial hospitalization as well as 30-day complications related to the index procedure (30). While there is some evidence that RC is safe with satisfactory patient outcomes at 3-year follow-up (31), there is limited reporting of long term outcomes. Our own experience performing RC over the last seven years has not demonstrated a significant difference in outcomes compared to LC.
Other studies have gone one step further to suggest that robotic assistance in a variety of general surgical procedures may eliminate the differences between hospitals and physicians (32). Biliary tract complications with cholecystectomy have a major impact on patient morbidity and financial costs (33,34). The use of robotics may standardize the safety of LC and improve associated morbidity and costs.
As robotic surgery continues to progress, the benefit from a patient-centered focus must also be considered in surgical decision making. Patient concerns for cosmesis and post-operative pain contributed to the shift from traditional open cholecystectomy to LC. In a further expansion of this trend, SSRC offers a better cosmetic result and has been demonstrated in a randomized control trial to have equivalent clinical outcomes and complications to traditional LC (35). Furthermore, the decreased numbers of trocar sites with SSRC leads to less pain and potential hernia sites. The incidence of hernia formation with SSRC will take several years to evaluate, but the likely benefits have significant clinical and financial implications.
Indocyanine green cholangiography
The SAGES Safe Cholecystectomy Program advocates structured steps to minimize the risk for bile duct injury during cholecystectomy (take away the capital letter) (36). The use of the da Vinci® robotic platform via either MPRC or SSRC allows adherence to all of these techniques. Although routine intra-operative cholangiography can also be performed with robotics, ICGC provides similar information in terms of verifying anatomy without the added time and cost of doing an additional procedure.
Indocyanine green (ICG) binds to plasma proteins in the blood and within the time frame of minutes, circulates through organs in the body before it is eliminated in the liver via the biliary system (37). The da Vinci® 3D optical system allows fluorescent imaging with its near-infrared imaging capability. Though the role of routine ICGC on common bile duct injury on a larger scale has yet to be evaluated, surgeons using this technology have demonstrated that it is safe with no ductal injuries reported in their clinical practices (38). Several studies have demonstrated equal or improved visualization of biliary anatomy and anatomic variants with ICGC, without the added time and morbidity associated with traditional intra-operative cholangiography (39,40). In addition, the conversion to open procedures has been decreased with the utilization of this technology (41). Use of direct ICGC with injection into the biliary tract is under evaluation and may have the same impact on RC safety and outcomes (42). The improved safety profile, enhanced visualization of bile duct anatomy, and decreased rates of conversion to open surgery have a significant impact on patient outcomes and downstream implications for surgeons from a systems perspective.
Robotic cholecystectomy in the face of new technologies
Current and developing technologies will impact outcomes of RC. Simulation platforms and true single orifice robotic platforms will change the progression of RC. These technologies may shift the balance of clinical outcomes to favor RC in the near future. The question of whether robotics should play a role in cholecystectomy may shift to whether laparoscopic cholecystectomy should still be performed.
Virtual reality training has been demonstrated to enhance operative skills of trainees (43). The da Vinci Skills Simulator® has been shown to provide similar training for the same physical skills on a standard robotic system (44). As the emphasis on simulation changes from simple task performance to procedure-based duplication of clinical scenarios, this will further reduce or eliminate the learning curve associated with the technology and improve operative safety. The ability to use imaging data to create pre-operative simulation of cholecystectomy anatomy may decrease the potential for biliary tract injury and increase the chances for completing procedures via the minimally invasive approach. This change in outcomes will have significant downstream implications on hospital performance and patient satisfaction. The increased morbidities of open surgery and biliary tract injury thereby will be alleviated (45).
The next generation of robotic platforms from both Intuitive Surgical and Titan Medical may bring a true single port device to the clinical arena that changes the current approach to robotic surgery. These platforms will challenge the current outcomes of RC and may demonstrate similar if not better outcomes. The analysis of these platforms will again look for a benchmark to make a comparison. The comparisons to open, LC, single-site LC, MPRC and SSRC will take many years and trials to define. This analysis will parallel the current environment with paralysis in decision making given the complexity of treatment options and limited large scale data. As additional robotic platforms reach clinical use the analysis of cost and effectiveness will increase in complexity as these platforms change what the “cost “of robotic surgery becomes. The complexity of data analysis will increase with increasing time to generate defined data.
Robotic cholecystectomy: the canary in the coal mine
Adoption of RC via either MPRC or SSRC has been limited in certain regions of the United States but has been widely adopted in other areas. There have been differences in utilization in academic versus larger hospital systems. These variations seem inconsistent and are not beneficial for patient care. As discussed in this review, robotic surgery can be cost neutral or cost effective as robotic volume increases and matches or exceeds outcomes from the current standard of care, LC. The use of this technology builds the robotic skill set required for more complex surgical procedures. The robotic platform is inherently safe, allowing 3D visualization and ICG fluorescence. Recognition of the value robotic surgery has been variable among administrators tasked to analyze the impact of this surgical technology. Outcomes in the literature for robotic-assisted procedures have been inconsistent and further muddy the water. Mature data points from established learning curves will emerge in the next 3–5 years and demonstrate clear evidence of superiority of RC. This superiority will most likely be present for both complex operations as well as simple operations like the cholecystectomy. This evidence is becoming apparent for other robotic operations, such as the incisional and inguinal hernia repairs. These “canary” procedures have been recognized by certain surgeons and administrators thereby demonstrating the difference in adoption of RC.
Hospitals that currently have robotic platforms can begin to see the benefits of RC without significant investments. The savings in both cost and time will facilitate further robotic procedures which again have additional benefits of decreased hospital length of stay, reduced morbidity and improved patient satisfaction. These downstream benefits are difficult to quantitate but are consistently present, thereby improving the institution’s reputation and financial situation. Furthermore, hospitals must acknowledge that recruiting quality surgeons without a robotic program may be difficult.
In the future, the use of robotic surgery for cholecystectomy is likely to become the standard of care when considering patient outcomes and satisfaction with quality of care. The current lack of full penetration is largely due to a cost-accounting method that is often arbitrary and is not reflected in conventional laparoscopic procedures. As a more direct method of calculating costs emerges, the utility of RC will become more universally recognized.
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr. Singh is a proctor for Intuitive Surgical.
References
- Hockstein NG, Gourin CG, Faust RA, et al. A history of robots: from science fiction to surgical robotics. J Robot Surg 2007;1:113-8. [Crossref] [PubMed]
- Reynolds W Jr. The first laparoscopic cholecystectomy. JSLS 2001;5:89-94. [PubMed]
- Satava RM. Surgical robotics: the early chronicles: a personal historical perspective. Surg Laparosc Endosc Percutan Tech 2002;12:6-16. [Crossref] [PubMed]
- Lanfranco AR, Castellanos AE, Desai JP, et al. Robotic surgery: a current perspective. Ann Surg 2004;239:14-21. [Crossref] [PubMed]
- Surgical I. The da Vinci Surgical System. Available online: http://www.intuitivesurgical.com/
- Nguyen NT, Nguyen B, Shih A, et al. Use of laparoscopy in general surgical operations at academic centers. Surg Obes Relat Dis 2013;9:15-20. [Crossref] [PubMed]
- He J, Amini N, Spolverato G, et al. National trends with a laparoscopic liver resection: results from a population-based analysis. HPB (Oxford) 2015;17:919-26. [Crossref] [PubMed]
- Rosales-Velderrain A, Stauffer JA, Bowers SP, et al. Current status of laparoscopic distal pancreatectomy. Minerva Gastroenterol Dietol 2012;58:239-52. [PubMed]
- Ecker BL, Datta J, McMillan MT, et al. Minimally invasive gastrectomy for gastric adenocarcinoma in the United States: Utilization and short-term oncologic outcomes. J Surg Oncol 2015;112:616-21. [Crossref] [PubMed]
- Badani KK, Bhandari A, Tewari A, et al. Comparison of two-dimensional and three-dimensional suturing: is there a difference in a robotic surgery setting? J Endourol 2005;19:1212-5. [Crossref] [PubMed]
- Moorthy K, Munz Y, Dosis A, et al. Dexterity enhancement with robotic surgery. Surg Endosc 2004;18:790-5. [Crossref] [PubMed]
- Tillou X, Collon S, Martin-Francois S, et al. Robotic Surgery Simulator: Elements to Build a Training Program. J Surg Educ 2016;73:870-8. [Crossref] [PubMed]
- Kim HJ, Choi GS, Park JS, et al. Comparison of surgical skills in laparoscopic and robotic tasks between experienced surgeons and novices in laparoscopic surgery: an experimental study. Ann Coloproctol 2014;30:71-6. [Crossref] [PubMed]
- Surgical I. Annual Report 2015. 2015.
- Barbash GI, Glied SA. New technology and health care costs--the case of robot-assisted surgery. N Engl J Med 2010;363:701-4. [Crossref] [PubMed]
- Breitenstein S, Nocito A, Puhan M, et al. Robotic-assisted versus laparoscopic cholecystectomy: outcome and cost analyses of a case-matched control study. Ann Surg 2008;247:987-93. [Crossref] [PubMed]
- Strosberg DS, Nguyen MC, Muscarella P 2nd, et al. A retrospective comparison of robotic cholecystectomy versus laparoscopic cholecystectomy: operative outcomes and cost analysis. Surg Endosc 2017;31:1436-41. [Crossref] [PubMed]
- Bedeir K, Mann A, Youssef Y. Robotic single-site versus laparoscopic cholecystectomy: Which is cheaper? A cost report and analysis. Surg Endosc 2016;30:267-72. [Crossref] [PubMed]
- Bibi S, Rahnemai-Azar AA, Coralic J, et al. Single-Site Robotic Cholecystectomy: The Timeline of Progress. World J Surg 2015;39:2386-91. [Crossref] [PubMed]
- Tsuda S, Oleynikov D, Gould J, et al. SAGES TAVAC safety and effectiveness analysis: da Vinci (R) Surgical System (Intuitive Surgical, Sunnyvale, CA). Surg Endosc 2015;29:2873-84. [Crossref] [PubMed]
- Himpens J, Leman G, Cadiere GB. Telesurgical laparoscopic cholecystectomy. Surg Endosc 1998;12:1091. [Crossref] [PubMed]
- Chitwood WR Jr, Nifong LW, Chapman WH, et al. Robotic surgical training in an academic institution. Ann Surg 2001;234:475-84; discussion 84-6. [Crossref] [PubMed]
- Kim VB, Chapman WH, Albrecht RJ, et al. Early experience with telemanipulative robot-assisted laparoscopic cholecystectomy using da Vinci. Surg Laparosc Endosc Percutan Tech 2002;12:33-40. [Crossref] [PubMed]
- Jayaraman S, Davies W, Schlachta CM. Getting started with robotics in general surgery with cholecystectomy: the Canadian experience. Can J Surg 2009;52:374-8. [PubMed]
- Vidovszky TJ, Smith W, Ghosh J, et al. Robotic cholecystectomy: learning curve, advantages, and limitations. J Surg Res 2006;136:172-8. [Crossref] [PubMed]
- Honaker MD, Paton BL, Stefanidis D, et al. Can robotic surgery be done efficiently while training residents? J Surg Educ 2015;72:377-80. [Crossref] [PubMed]
- Juza RM, Haluck RS, Won EJ, et al. Training current and future robotic surgeons simultaneously: initial experiences with safety and efficiency. J Robot Surg 2014;8:227-31. [Crossref] [PubMed]
- Angus AA, Sahi SL, McIntosh BB. Learning curve and early clinical outcomes for a robotic surgery novice performing robotic single site cholecystectomy. Int J Med Robot 2014;10:203-7. [Crossref] [PubMed]
- Su WL, Huang JW, Wang SN, et al. Comparison study of clinical outcomes between single-site robotic cholecystectomy and single incision laparoscopic cholecystectomy. Asian J Surg 2017;40:424-8. [Crossref] [PubMed]
- Maeso S, Reza M, Mayol JA, et al. Efficacy of the Da Vinci surgical system in abdominal surgery compared with that of laparoscopy: a systematic review and meta-analysis. Ann Surg 2010;252:254-62. [Crossref] [PubMed]
- Bodner J, Hoeller E, Wykypiel H, et al. Long-term follow-up after robotic cholecystectomy. Am Surg 2005;71:281-5. [PubMed]
- Altieri MS, Yang J, Telem DA, et al. Robotic-assisted outcomes are not tied to surgeon volume and experience. Surg Endosc 2016;30:2825-33. [Crossref] [PubMed]
- Worth PJ, Kaur T, Diggs BS, et al. Major bile duct injury requiring operative reconstruction after laparoscopic cholecystectomy: a follow-on study. Surg Endosc 2016;30:1839-46. [Crossref] [PubMed]
- Andersson R, Eriksson K, Blind PJ, et al. Iatrogenic bile duct injury--a cost analysis. HPB (Oxford) 2008;10:416-9. [Crossref] [PubMed]
- Kudsi OY, Castellanos A, Kaza S, et al. Cosmesis, patient satisfaction, and quality of life after da Vinci Single-Site cholecystectomy and multiport laparoscopic cholecystectomy: short-term results from a prospective, multicenter, randomized, controlled trial. Surg Endosc 2017;31:3242-50. [Crossref] [PubMed]
- Pucher PH, Brunt LM, Fanelli RD, et al. SAGES expert Delphi consensus: critical factors for safe surgical practice in laparoscopic cholecystectomy. Surg Endosc 2015;29:3074-85. [Crossref] [PubMed]
- Kim KC. Robotics in general surgery. New York: Springer, 2013.
- Daskalaki D, Fernandes E, Wang X, et al. Indocyanine green (ICG) fluorescent cholangiography during robotic cholecystectomy: results of 184 consecutive cases in a single institution. Surg Innov 2014;21:615-21. [Crossref] [PubMed]
- Osayi SN, Wendling MR, Drosdeck JM, et al. Near-infrared fluorescent cholangiography facilitates identification of biliary anatomy during laparoscopic cholecystectomy. Surg Endosc 2015;29:368-75. [Crossref] [PubMed]
- Vlek SL, van Dam DA, Rubinstein SM, et al. Biliary tract visualization using near-infrared imaging with indocyanine green during laparoscopic cholecystectomy: results of a systematic review. Surg Endosc 2017;31:2731-42. [Crossref] [PubMed]
- Gangemi A, Danilkowicz R, Elli FE, et al. Could ICG-aided robotic cholecystectomy reduce the rate of open conversion reported with laparoscopic approach? A head to head comparison of the largest single institution studies. J Robot Surg 2017;11:77-82. [Crossref] [PubMed]
- Liu YY, Kong SH, Diana M, et al. Near-infrared cholecysto-cholangiography with indocyanine green may secure cholecystectomy in difficult clinical situations: proof of the concept in a porcine model. Surg Endosc 2016;30:4115-23. [Crossref] [PubMed]
- Seymour NE, Gallagher AG, Roman SA, et al. Virtual reality training improves operating room performance: results of a randomized, double-blinded study. Ann Surg 2002;236:458-63; discussion 63-4. [Crossref] [PubMed]
- Brown K, Mosley N, Tierney J. Battle of the bots: a comparison of the standard da Vinci and the da Vinci Surgical Skills Simulator in surgical skills acquisition. J Robot Surg 2017;11:159-62. [Crossref] [PubMed]
- Hogan NM, Dorcaratto D, Hogan AM, et al. Iatrogenic common bile duct injuries: Increasing complexity in the laparoscopic era: A prospective cohort study. Int J Surg 2016;33 Pt A:151-6.


