The natural history of nonalcoholic fatty liver disease: mortality rates and liver enzymes
Nonalcoholic fatty liver disease (NAFLD) has emerged as an important health issue of the modern world, due to the dramatic increase in its prevalence, which has doubled and in some regions tripled in the past decade (1).
Epidemiological studies, including national health surveys, are remarkable instruments for indirectly measuring the health status of the general population, as well as providing estimations of the mortality and morbidity associated with major diseases and delineating global risk factors. Recently, Unalp-Arida and Ruhl attempted to establish whether NAFLD is associated not only with increased liver disease mortality, but overall mortality rates in the US. (2). In addition, they explored the role of non-invasive biomarkers in the prediction of the natural history of NAFLD, including mortality rates. For this purpose, the authors retrieved data from the National Health and Nutrition Examination Survey (NHANES) I–III (1988–2011), which included up to 23 years of linked-mortality data. As a part of their study, Unalp-Arida and Ruhl analyzed the Survey-linked National Death Index records of 14,527 adults who were negative for viral hepatitis B and C, as well as iron overload. Hepatic steatosis (HS) was measured by liver ultrasound and classical liver function test that were used as surrogate indicators of NAFLD. The sample comprised of 7,739 participants without and 4,494 with NAFLD, 923 of whom were classified as having “severe” HS based on imaging parameters. Of note, the authors reported 36.2% all-causes cumulative mortality rate, 16.3% and 1.1% of which were attributed to cardiovascular disease (CVD) and liver disease, respectively (2). The results pertaining to patients with severe NAFLD are summarized in Figure 1. These individuals also had increased body mass index (BMI) and waist-to-hip ratio, higher systolic and diastolic blood pressure, increased prevalence of type 2 diabetes (T2D) and arterial hypertension, elevated C-reactive protein, and increased levels of plasma total cholesterol (Figure 1). These results reinforce the concept of a shared genetic effect between NAFLD and the metabolic syndrome (MetS), whereby ~44% of genes associated with NAFLD are probably shared by other MetS components, including T2D, obesity and hypertension combined (3). As pointed out by Unalp-Arida and Ruhl, the PNPLA3 p.I148M variant is one of the most extensively studied genetic influences, which explains more than 5% of the liver fat variance and NAFLD severity (4,5). Another variant associated with NAFLD, TM6SF2 p.E167K, is also of interest, as the E-allele, which has an overall frequency of up to 93%, is associated with higher blood lipids, while the K-allele confers risk for NAFLD (6). However, as expected for complex diseases, the authors found that exposure to environmental factors, such as increased alcohol consumption and decreased physical activity, was significantly different among subjects with and without-NAFLD. These results highlight recent knowledge gained from, for instance, mendelian randomization studies suggesting that alcohol consumption—even in light or moderate amounts—increases the risk and severity of NAFLD (7).
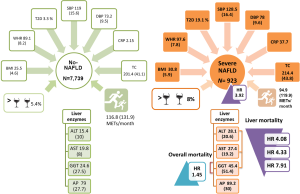
Finally, severe HS was associated with ~4-fold risk of mortality for liver-related diseases (2), even if specific causes of death could not be clearly described. Surprisingly, HS was not independently associated with mortality rates of CVD, T2D or even cancer (2). This lack of association can be explained by the fact that adjusting for comorbidities, which cluster together in the MetS, may produce overfitting. It would thus be interesting to examine the degree of tolerance among co-variables in the adjusted model.
Liver enzymes and mortality rates: shooting the messenger
Unalp-Arida and Ruhl have also observed that liver enzymes, including alanine (ALT) and aspartate (AST) aminotransferases, and gamma-glutamyltransferase (GGT) were associated with increased risk of liver disease-related mortality (Figure 1). The hazard ratio (HR) for the levels of these enzymes was reported to range from 4.08 to 7.91, whereby the lowest values were found for ALT, followed by AST, and the highest for GGT. These interesting results prompt several questions. For instance, are aminotransferases merely markers of liver injury or are they indeed surrogate indicators of systemic alterations?
The use of liver ultrasound and the measurement of serum levels of aminotransaminases, which have been—if mildly elevated—classically regarded as surrogate indicators of serious disease, are both frequently used for the diagnosis of NAFLD in epidemiological studies or screening programs. Nevertheless, it is known that the entire histologic spectrum of NAFLD, including the severe clinical picture of nonalcoholic steatohepatitis (NASH) with advanced fibrosis, can be seen in individuals with normal values of ALT or AST (8).
Recent explorations on liver gene and protein expression changes of aminotransferases, including their corresponding isoforms, in patients with NAFLD have introduced a new paradigm into the biological role of liver aminotransferases in the regulation of the systemic metabolic functioning (9). In fact, we have shown that all the intermediate phenotypes of the MetS are associated with perturbed liver gene/protein expression levels of aminotransferases, as well as with circulating metabolites derived from the Krebs cycle (9). Therefore, rather than a simple biomarker of hepatocyte injury, aminotransferases might be regarded as sensors of global metabolic deregulation, including mitochondrial energetic control (9).
Taken together, these findings suggest that the levels of aminotransferases are sensors of metabolic complications, rather than serving merely as biomarkers of liver necrosis. This assertion is supported by Unalp-Arida and Ruhl, who demonstrated that ALT levels in the highest decile are associated with over twice the risk of mortality in age-adjusted analysis (2), indicating the need for exploring this phenomenon through further cohort studies. Likewise, the results showing the levels of AST in the highest decile also associated with unadjusted all-causes cumulative mortality rates (2) should be considered for further analysis of the subsequent NHANES-surveys.
GGT: antioxidant or pro-oxidant biomarker?
Unalp-Arida and Ruhl found serum GGT elevation to be related to all-causes mortality (2), as subjects with elevated GGT levels had a 1.45-HR of mortality (Figure 1).
Serum levels of GGT have been traditionally used as markers of liver injury, particularly chronic cholestatic diseases and liver tumors, as well as alcohol consumption (10). Nevertheless, robust epidemiological evidence indicates that serum GGT levels are associated with vascular disease (11) and all the components of the MetS (10), as well as with kidney disease and cancer. Specifically, a recent meta-analysis of 35 studies including 571,511 controls and 72,196 cases showed that serum GGT levels are associated with increased all-causes mortality, as well as cardiovascular and cancer-related mortality (12). What exactly is the biological meaning of these epidemiological findings?
GGT is a membrane-bound enzyme of the external cell surface that catalyzes the hydrolysis of gamma-glutamyl bonds in gamma-glutamyl compounds such as glutathione, its most abundant substrate. In fact, GGT initiates extracellular glutathione (GSH) breakdown, providing cells with local cysteine supply and contributing to the maintenance of the intracellular GSH levels.
GGT is expressed not only in the liver but also in many different tissues, including kidneys, spleen, gut and acinar pancreatic cells, among others; nevertheless, the enzymatic activity varies considerably across normal tissues (10). Of note, the expression of GGT in the liver is ~20% of that in kidneys (10). Multiple alternatively spliced variants of GGT have been identified; there are also a number of related genes present on chromosomes 20 and 22, as well as putative pseudogenes for GGT gene on chromosomes 2, 13, and 22.
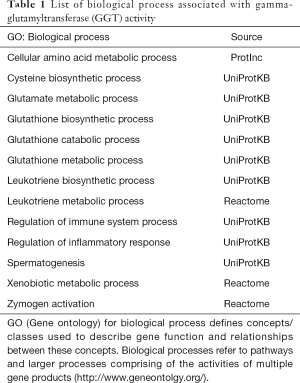
On the bases of the biological processes in which GGT is involved (Table 1), it has been suggested that this enzyme plays an important role in protecting cells against oxidative stress. This concept is also supported by the effect/s of GGT deficiency observed in experimental models, including knockout mice (10).
Full table
However, authors of some extant studies have pointed out the potential involvement of GGT in the production of reactive oxidative species (ROS) by inducing NF-kB-binding (13). In fact, it was shown that, in the presence of molecular oxygen and iron or copper ions, GGT might be involved in the generation of ROS (14). The high GGT activity of preneoplastic hepatic foci produced in rats by chemical carcinogens would support this concept (10).
Regardless of whether GGT is pro- or anti-antioxidant, it is clear that serum levels of this enzyme provide information on pathways that are relevant for the cellular homeostasis. Moreover, serum GGT levels are remarkable surrogate of disease-associated pathways, including regulation of inflammatory and immune system processes, and xenobiotic metabolism, as shown in Table 1.
On the other hand, the synthesis of GSH from glutamate, cysteine, and glycine also highlights the intricate metabolic derangement associated with NAFLD and the MetS, which involves branched-chain amino acids and glutamine (15). It is thus not surprising that, while GSH-associated pathways occur in virtually all cell types, the liver is the major organ in both GSH production and its exporting. Hence, circulating GGT levels may be reliable surrogate indicators of cellular stress, whereby they either denote abnormal turnover rates of GSH or high levels of enzymatic induction of GGT as a mechanism protecting the cell against an imbalance in GSH levels (10). When these findings are considered jointly, it is plausible to assume that GGT serum levels provide information on the whole body stress-associated homeostasis; consequently, elevated GGT levels in the serum correlate with the incidence and prevalence of a myriad of common diseases, including CVD and cancer, and thereby with the risk of all-cause mortality.
In summary, the results reported here are truly demonstrative of the complexity of the natural history of NAFLD, which would without any intervention progress to a more detrimental form and thus requires immediate intervention.
Acknowledgements
Funding: This study was partially supported by grants PICT 2014-0432, PICT 2014-1816 and PICT 2015-0551 (Agencia Nacional de Promoción Científica y Tecnológica, FONCyT). SS and CJP belong to Consejo Nacional de Investigaciones Científicas (CONICET).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Brunt EM, Wong VW, Nobili V, et al. Nonalcoholic fatty liver disease. Nat Rev Dis Primers 2015;1:15080. [Crossref] [PubMed]
- Unalp-Arida A, Ruhl CE. Noninvasive fatty liver markers predict liver disease mortality in the U.S. population. Hepatology 2016;63:1170-83. [Crossref] [PubMed]
- Sookoian S, Pirola CJ. Nonalcoholic fatty liver disease and metabolic syndrome: Shared genetic basis of pathogenesis. Hepatology 2016;64:1417-20. [Crossref] [PubMed]
- Sookoian S, Castaño GO, Burgueño AL, et al. A nonsynonymous gene variant in the adiponutrin gene is associated with nonalcoholic fatty liver disease severity. J Lipid Res 2009;50:2111-6. [Crossref] [PubMed]
- Sookoian S, Pirola CJ. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology 2011;53:1883-94. [Crossref] [PubMed]
- Pirola CJ, Sookoian S. The dual and opposite role of the TM6SF2-rs58542926 variant in protecting against cardiovascular disease and conferring risk for nonalcoholic fatty liver: A meta-analysis. Hepatology 2015;62:1742-56. [Crossref] [PubMed]
- Sookoian S, Flichman D, Castaño GO, et al. Mendelian randomisation suggests no beneficial effect of moderate alcohol consumption on the severity of nonalcoholic fatty liver disease. Aliment Pharmacol Ther 2016;44:1224-34. [Crossref] [PubMed]
- Mofrad P, Contos MJ, Haque M, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology 2003;37:1286-92. [Crossref] [PubMed]
- Sookoian S, Castaño GO, Scian R, et al. Serum aminotransferases in nonalcoholic fatty liver disease are a signature of liver metabolic perturbations at the amino acid and Krebs cycle level. Am J Clin Nutr 2016;103:422-34. [Crossref] [PubMed]
- Whitfield JB. Gamma glutamyl transferase. Crit Rev Clin Lab Sci 2001;38:263-355. [Crossref] [PubMed]
- Fraser A, Harris R, Sattar N, et al. Gamma-glutamyltransferase is associated with incident vascular events independently of alcohol intake: analysis of the British Women's Heart and Health Study and Meta-Analysis. Arterioscler Thromb Vasc Biol 2007;27:2729-35. [Crossref] [PubMed]
- Long Y, Zeng F, Shi J, et al. Gamma-glutamyltransferase predicts increased risk of mortality: a systematic review and meta-analysis of prospective observational studies. Free Radic Res 2014;48:716-28. [Crossref] [PubMed]
- Accaoui MJ, Enoiu M, Mergny M, et al. Gamma-glutamyltranspeptidase-dependent glutathione catabolism results in activation of NF-kB. Biochem Biophys Res Commun 2000;276:1062-7. [Crossref] [PubMed]
- Drozdz R, Parmentier C, Hachad H, et al. gamma-Glutamyltransferase dependent generation of reactive oxygen species from a glutathione/transferrin system. Free Radic Biol Med 1998;25:786-92. [Crossref] [PubMed]
- Sookoian S, Pirola CJ. Alanine and aspartate aminotransferase and glutamine-cycling pathway: their roles in pathogenesis of metabolic syndrome. World J Gastroenterol 2012;18:3775-81. [Crossref] [PubMed]


