Value-based assessment of robotic pancreas and liver surgery
“The failure to prioritize value improvement in health care delivery and to measure value has slowed innovation” (1).
Introduction to value-based analysis
Hospital economic analysis is often limited to a simple accounting of cost from the perspective of a single stakeholder to the healthcare delivery process (1). Broader concepts of cost, such as cost-effectiveness, cost-benefit ratio, and cost-minimization, add outcomes to the cost equation but are limited formulations of “value”. The Institute of Medicine defined value as the ratio of benefits to cost in 2008 (2). True value-based decision analysis incorporates all outcomes impacted by a single episode of care as well as effects on stakeholder groups invested in the delivery process, such as patients, doctors, hospitals, and payers. This method of economic analysis is essential to analyze the relative value of new technologies introduced into the healthcare marketplace.
Robot-assisted minimally-invasive surgery heralds a new era of technology application in surgery with multiple long-term innovations converging into a new instrument platform. The current generation of robots combines image-guidance, computer-aided dexterity controlling more than two effector arms, three-dimensional vision in both visible and non-visible spectra, new energy and stapling devices, and remote surgery into one device. Merging these 21st century technologies creates potential benefits and consequences extending well beyond the economic impact of these devices in regulated marketplace, but is nonetheless the subject of this brief overview.
The marketplace for robotic devices
Robotic surgery has evolved significantly since 1985 when the PUMA 560 was first used to direct a needle for brain biopsy (3). Within two years, the first robotic cholecystectomy (RC) was performed. Government-sponsored research efforts in the late 1980’s and early 1990’s contributed to further advances in robot-assisted surgery produced by Computer Motion, Inc. and Integrated Surgical Systems, companies which merged in 2003 to create Intuitive Surgical Inc. (ISI) (4).
ISI had an estimated market share of 80% in 2016 (5) and remains the sole supplier of instruments used in robot-assisted hepatopancreaticobiliary (HPB) surgery. Current ISI robots cost from $910,000 to $2.5 million USD with annual maintenance contracts of $125,000 (6). Exponential growth in technology and adoption of robotic surgery is predicted to increase sales of robotic instruments to 18 billion dollars annually within the next few years (7). Given this revenue opportunity, additional market entrants are emerging to compete for market share, including Titan Medical, TransEnterix, Medtronic, Verb, Stryker, Blue Belt Tech, and Acrobot (5). These companies may accelerate the pace of innovation, expand robotic approaches to other areas of surgery, and reduce prices.
Growth in robotic surgery reflects engineering solutions to the limitations of traditional laparoscopic approaches. These include extended range of motion with seven degrees of freedom; elimination of surgeon tremor allowing two-handed suturing and dissection; articulating staplers and energy devices to permit true four-quadrant resection of major organs; and high-definition three-dimensional vision. Additionally, many surgeons prefer the ergonomics of sitting at an adjustable console rather than holding laparoscopic instruments in an unsupported standing position.
Increasingly complex procedures have adopted minimal access techniques given accumulating evidence in multiple specialties that minimally invasive surgery reduces lengths of stay and improves postoperative quality of life (8,9). Evolving single and multi-institutional studies indicate that these benefits may extend to pancreas and liver surgery, a field of added complexity due to the inherent technical difficulty of these procedures combined with their low frequency and high morbidity (10). Robotic assistance may bridge the gap between open and minimally-invasive HPB surgery which impedes adoption by the majority of surgeons. Examples of technical capabilities enhanced by the robot include: control of bleeding by the mesenteric vessels (11), the potential for a positive surgical margin (12), and pancreatic fistula (13).
Whether these potential advantages provide sufficient additional value to justify the increased expenditure on robotic equipment remains an open question. Existing financial data are derived from simple cost models obtained from single institution retrospective data without a full accounting of financial cost or patient-centered benefit. From the standpoint of “cost”, adding a robot to the operative team entails both monetary and non-monetary expenses. First, there is a training period and flat learning curve before a surgeon can be considered proficient. Second, despite the added complexity and expense of each procedure, Current Procedural Terminology (CPT) codes have not provided reimbursement for robot-assisted procedures, meaning that surgeons are paid using standard payment schedules delayed by prolonged payer-level review for unlisted procedure codes. These cumulative hurdles have provided little economic incentive for the practicing HPB surgeon to innovate. The incremental cost side of the value equation is far easier to enumerate. Three factors dominate accounting for robotic surgery: amortization of acquiring the robot on a per case basis, the ongoing expense of disposable instrumentation, and the annual maintenance contract. All of these costs are addressed by Intuitive’s original FY2000 SEC filing of form 10-K: Intuitive proposes a bold business plan to use the computer-enforced service life of its instruments to maintain its control over costs on a “per-procedure or per-hour basis” (14). This unique approach to revenue generation is a temporary aberration created by the market dynamics of a single supplier of robotic devices and has no long term effect on the value of robot-assisted surgery in a competitive marketplace.
The stakeholder approach to value
Before examining the costs of existing robotic devices, we must first define the value proposition of robotic surgery for stakeholders participating in the provision of health care services: patients, surgeons, hospitals, and payers.
Patient-centered value
Patients assess value per the following equation:
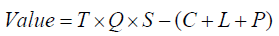
where: T = change in overall survival, Q = change in quality of life, S = satisfaction with outcomes, C = care costs, L = income loss, and P = premium payment.
This measure of patient value highlights critical discrepancies between the perceptions of physicians and patients in the healthcare system. Physicians do not always appreciate the patient’s value proposition, which is critical to providing quality care. Although overall survival and quality of life are the two most important outcome measures for patients, only overall survival can be measured directly. There are numerous validated instruments for measuring quality of life, but no agreement has been reached about a single tool to evaluate surgical outcomes. Physicians, patients, caregivers, and family members frequently reach different conclusions about quality of life as measured by history and physical examination (15). Multiple tools have been formulated to assess quality of life and can generally be broken down into generic, disease-specific, and symptom severity assessments. Some experts suggest that two quality of life assessments are required to compare interventions: a general assessment, like SF-36, and a second disease or procedure-specific assessment. The variety of tools in current use makes comparing quality of life outcomes between institutions and procedures impossible and prevents direct cost-utility analysis among published studies.
Patient satisfaction further complicates the calculation of value to achieve high-quality health care from the patient’s point of view. Although patients are an effective indicator of the type of care they receive (16), there is currently no agreed standard for measuring patient satisfaction.
Costs are similarly difficult to quantitate due to complexities built into the payer formula whereby patient costs are divided into co-pays, deductibles, and insurance premium payments. Whereas cost directly affects the patient’s value proposition, simple accounting often ignores opportunity costs associated with alternate treatment strategies, especially minimally-invasive techniques designed to expedite return to work. Income loss caused by surgery and recovery are not publicly reported and vary significantly between individual patients. Whereas health, disability, and life insurance companies all deal with these complex income losses, they have little incentive to share information with each other to formulate a detailed analysis of income loss.
The surgeon value proposition
Second only to patients, surgeons have the largest input to the total value equation for robotic HPB surgery. The formulation of surgeon value in a single episode of care is:

where I = income generated, K = knowledge gained, R = change in reputation, C = prorated practice costs, L = opportunity loss, and E = effort expended. As this formula demonstrates, surgeon value may be affected by self-interest without the check of outcome transparency in the current system. Moreover, income, prorated practice costs, and opportunity loss may be indirectly linked to the hospital balance sheet. Most studies group total hospital expense and surgeon income and costs in the same category, a flawed assumption in the stakeholder analysis since surgeons have interests in the process of innovation that may diverge from those of the hospital.
While there is no direct measure of knowledge gained by the surgeon, the most closely aligned endpoint is total operative time. Shakir et al. published their experience of adopting robotic surgery techniques and measured progress using CUSUM analysis of operative time, readmissions, morbidities, and length of stay (17). This analysis demonstrated that the learning curve to master a new procedure can be prolonged and flat, raising the activation energy for surgeons to perform robotic HPB cases without any corresponding incentive through existing CPT reimbursement codes.
Reputation and effort are similarly important drivers of the surgical value equation. 92% of modern consumers read physician reviews online, a frequency second only to restaurant reviews. Surgeon reputation is therefore a key but under-recognized factor driving episodes of surgical care (18), probably second only to the effort expended by the surgeon during the procedure. One of the major factors limiting the number of cases performed by a surgeon in a day is the duration of conventional open or laparoscopic procedures spent in ergonomically uncomfortable positions (19). Vidovszky et al. suggested that the ergonomics of robotic surgery diminishes fatigue and reduces surgeon injuries relative to long hours holding laparoscopic instruments in unsupported positions (20). Jensen et al. speculate that robotic surgery may allow surgeons to delay retirement, reducing expenses associated with attrition, training and (21), saving up to one million dollars invested into surgical training.
The hospital value proposition
Hospitals are vital to the healthcare delivery process both operationally and financially. Value in the hospital setting is expressed as:
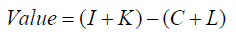
where I = income generated, C = costs to render care (which can be subdivided into fixed and variable costs), L = opportunity loss, K = knowledge gained.
A hospital’s reputation is a major contributor to its value proposition. Hospitals regularly publicize ratings from US News and World Report to influence public opinion about the quality of care, even though the correlation between hospital ranking and quality is likely poor. Chau et al. evaluated hospital ranking systems and associated outcomes of 804 pancreatectomies performed at eleven hospitals and found that hospital rankings were not correlated with actual outcomes, confounding evaluations of quality by patients and providers seeking pancreatic cancer surgery (22).
During the process of new technology evaluation, hospitals continually face “opportunity loss” defined as the cost of lost opportunity because a different choice was made. Denials of insurance coverage are the leading source of opportunity loss and impact 8.1% of all initial claims (23). Other measures of opportunity loss include lost operating room time which could be used to treat more patients and increase profits. The opportunity cost of robotic surgery requires case-specific examination. For example, longer operative times have been reported for robot-assisted liver surgery (24), whereas robotic distal pancreatectomy was performed faster than the laparoscopic approach (25). No published data assesses the knowledge gained by the entire staff during a single operative procedure. The only modest measure of this phenomenon is the docking time for the Da Vinci robot, a metric that clearly improves with case experience (26).
Value for payers
The insurance industry calculates total value as premiums generated (P) minus business costs (C), summarized as:
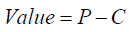
Because insurance companies do not participate directly in surgery, their input to the total value equation is their income and financial payout. These multi-billion dollar organizations hire statisticians to determine whether given services or patients should be covered and employ lawyers to deal with litigation over complex insurance policies under the cover of ERISA, which largely shields employer-sponsored health plans from liability (27).
The financial implications of robot-assisted HPB surgery
There is limited and conflicting data regarding costs for robotic HPB surgery. Most analyses focus on total hospital costs or patient charges but do not analyze net hospital income. Costs are often divided into operative and non-operative costs, and subdivided per specialty and service, such as nursing, radiology, or anesthesia. In the case of robotic surgery, the direct expenses include the acquisition costs of the robot amortized over the predicted case volume, the recurring expenses of instrument purchase and maintenance, minus the potential indirect savings associated with reduced length of stay and improved outcomes and reputation that result.
Economics of major pancreatic resection
The actual incremental cost of robot-assisted major pancreatic resection is unclear as the following economic analyses will demonstrate.
Robot-assisted distal pancreatectomy (RADP)
Five evaluations of robotic distal pancreatectomy include cost (Table 1). Waters et al. performed a single institution retrospective analysis of 77 robotic, open, and laparoscopic distal pancreatectomies (28). Although there was a trend toward lower median cost for robotic ($11,703) versus laparoscopic ($14,354) or open ($17,751) procedures, no statistically significant differences were observed. Despite the amortized cost of $1,316 per robotic procedure, the authors observed average savings of $6,048 compared to open distal pancreatectomy and $2,651 compared to the laparoscopic approach. Although the robotic system significantly increased direct operative costs (a form of variable costs), net cost savings were created by statistically significant reductions in length of stay after robotic surgery (4 days) compared to laparoscopic (6 days) or open surgery (8 days). The hospital does not actually cut costs significantly by reducing length of stay. In fact, most of a US hospital’s revenue comes from the first day or two of a patient’s admission under the current DRG system (Diagnosis Related Group). Rather, shortening length of stay reduces opportunity losses associated with occupied beds that can be reassigned to patients with higher-margin disease states. Although the authors concluded that robotic distal pancreatectomy was safe and cost-effective, the study demonstrated selection bias that affected length of stay and had a small sample size with a paucity of long-term follow-up costs.

Full table
On the other hand, Kang et al. compared robotic and laparoscopic distal pancreatectomy at a single institution in Korea (29) and concluded that robotic surgery was significantly more expensive ($8,898) than the conventional laparoscopic approach ($4,137). Direct operative costs drove up expenses in the robotic group without corresponding improvements in length of stay (7.3 vs. 7.1 days, respectively). Kang’s methodology to analyze cost was not clearly delineated and further excluded the costs to amortize the purchase of the robot or subdivide expenses associated with equipment, anesthesia, or pharmacy. Moreover cross national costs comparisons are difficult to compare, as are different payment structures, patient expectations and support structures.
Wayne et al. performed a retrospective comparison of patient charges during robotic and open distal pancreatectomies at a single high volume center during the 2013 fiscal year (30). Their study was limited by its small sample size (n=12), lack of statistical analysis, and exclusion of amortization costs. The authors reported higher operating room charges for robotic cases compared to open procedures ($2,255 to $1,810), and that differences between groups were only partially offset by reduced length of stay in the robotic group (3.9 vs. 6.5 days).
A prospective study by Butturini et al. investigated instrumentation costs at a single center in Europe (31). Limited data was reported regarding instrumentation cost. The average cost of robotic instruments for distal pancreatectomy was $3,330, twice that of the laparoscopic approach. The small sample size (n=43) and limited cost data prevented direct comparisons regarding cost.
Analysis of RADP
Four published studies analyzing distal pancreatectomy show little agreement. Only two studies examined total costs but reached contradictory conclusions (28,29). Two studies were prospective (29,31), but only one included amortized cost of the robot (28). The analysis of Waters et al. used an approach most analogous to value-based discussion but failed to demonstrate a statistically significant cost savings over laparoscopic and open approaches. Kang et al. found a statistically significant $4,800 increase in costs for robotic surgery. Analyses by Butturini et al. and Wayne et al. are less informative due to limitations imposed by restricting data to instrument and operating room costs. While much of the literature focuses on readily obtainable cost data, the true cost and value of robotic surgery cannot be solely evaluated using direct operating costs without accounting for opportunity losses caused by prolonged hospital stays after conventional open procedures or avoidable conversions to open surgery. For example, the event rate for conversion during laparoscopic distal pancreatectomies was 20% in the 2014 ACS-NSQIP data (32).
Robot-assisted pancreaticoduodenectomy (RAPD)
There have been only two studies evaluating the cost of robotic pancreaticoduodenectomy (Table 2). Baker et al. compared charges for robotic and open PD in a single institution retrospective analysis. Costs were subdivided into operating room, in-hospital, follow-up, and total costs (33). Operative charges for the robotic group ($51,402) were significantly higher than open ($32,857) due to longer operative times and increased usage of disposables and other equipment. Conversely, robotic patients’ experienced 72% fewer 30-day postoperative complications, 50% shorter ICU stays, and 22% shorter total hospital length of stay. These factors reduced inpatient charges relative to open leading to comparable median total charges (robot: $144,560; open: $153,025) and inpatient charges (robot: $143,982; open: $138,557). Key limitations to these data include imbalanced number of open (n=49) and robotic (22) procedures and exclusion of the amortization costs for purchasing the robot.

Full table
A prospective single institution study by Boggi et al. compared mean operative cost between the open and robotic approaches and also included cost calculations (34). Boggi et al. reported excess average operative cost of $6,917 per robotic case and concluded that high costs and prolonged operative times were a limitation of robotic pancreaticoduodenectomy.
Analysis of RAPD
There is insufficient evidence to assess the impact of robotic surgery on costs associated with pancreaticoduodenectomy. While operative costs are consistently higher for RAPD (33,34), neither the value nor the cost equation can be consistently determined compared to open because the comparative effectiveness of the two procedures remains unclear and the market for robot instruments is finally beginning to evolve. Additional short term analyses are not expected to demonstrate cost savings because robotic surgery is associated with excess equipment charges in a single supplier marketplace.
Rather, potential cost savings derive from speculative benefits resulting from shorter length of stay and reduced complications like surgical site infections. The opportunity losses of open PD are substantial. The average hospital expense for an inpatient day across the US in 2016 was $2,322 (35). A reduction in the surgical site infection rate as indicated by Baker et al. (33) would have an even greater benefit. A 2009 report by the Center for Disease Control, adjusted to current $USD, estimated the cost of a single surgical site infection to be between $11,732 and $28,701 (36).
Cost of robotic major liver resection
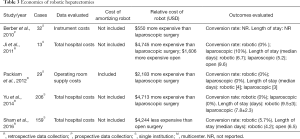
Five studies report the cost of robotic liver resection. Sham et al. performed a retrospective analysis of total hospital costs of 159 robotic and open liver resections (37). Excluding amortization, the robotic approach was significantly less expensive than open (savings: $14,754 to $18,998). When categorized by preoperative, operative, and postoperative charges, the robot was more expensive for both perioperative and operative services. However, postoperative costs favored the robot by $4,855, the result of a two-day reduction in hospital stay. The robotic group had significantly higher costs for pathology, ICU, labs, and other miscellaneous inpatient services, totaling $1,600 per case. Despite omitting amortization costs, the authors considered the robotic approach “cost-competitive”.
Yu et al. retrospectively compared robotic to laparoscopic hepatectomy at a single Korean institution and reported total hospital costs excluding amortization (38). The robotic cohort had significantly higher costs than the laparoscopic approach (robot: $11,475; lap: $6,762). The length of stay trend favored the laparoscopic group (9.5 vs. 7.8 days).
Another single-institution retrospective analysis of robotic and laparoscopic hepatic resections reported cost data for operating room supply costs (39). Although supply costs were initially similar between groups, robotic resections were more expensive after amortizing $1,448 per robotic case. Further, the robot did not reduce length of stay compared to the laparoscopic group (4 vs. 3 days, respectively). Although this analysis was limited by incomplete hospital data and cost breakdown analysis, the authors concluded that the robotic approach yielded slightly inferior outcomes at increased cost compared to laparoscopic techniques.
Ji et al. performed a retrospective analysis of 13 robotic, 20 laparoscopic, and 32 open hepatectomies at a single Chinese institution and reported total hospital costs without amortization (40). The robotic cohort had significantly higher hospital costs than both laparoscopic and open approaches (robot: $12,908; laparoscopic: $8,163; open: $11,302). The authors attributed higher costs to instrumentation costs and longer operating room times. Although the robotic cohort had the shortest length of stay, the study failed to reach any net conclusion on cost-effectiveness and suggested more data were needed.
Finally, Berber et al. examined robotic hepatectomy in comparison with laparoscopic techniques (41). The analysis did not include any case-by-case or total cost analysis. The only data on cost was the general statement that the robotic approach “[generally] adds $550 per case to the laparoscopic equipment cost”.
Analysis of robotic major hepatectomy
Five studies evaluated robotic hepatectomy compared to open and demonstrated no net reduction in cost (Table 3). Three of the five studies reported total hospital costs; two found higher costs for the robotic approach (38,40) and one found lower costs (37). The surgical approach had no consistent effect on length of stay. Robotic liver resection added between $1,500–$5,000 per procedure to total hospital costs as compared with standard laparoscopic techniques. These data were limited by case selection bias, as none of the important variables governing the selection for robotic, laparoscopic, or open cohorts was evaluable. Furthermore, none of the cost data were calculated from an intent-to-treat perspective, as the major cost savings likely accrue among patients for whom a minimally-invasive approach can be successfully completed. This is the major cost consideration given the relationship between complication rates and total costs. The robot may be a tool that permits more surgeons to complete a minimally-invasive procedure with fewer conversions to open compared with the laparoscopic procedure.
Full table
RC
Among operations in the HPB realm, cholecystectomy is the most common but also the least complex. The laparoscopic approach is currently considered the gold standard. The emergence of robot-assisted technology requires an analysis of the relative merits of the two approaches (Table 4).
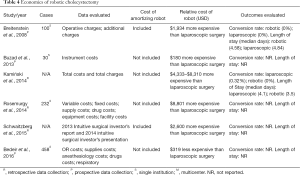
Full table
Breitenstein prospectively evaluated 50 robotic and 50 laparoscopic cholecystectomies at a single institution in 2008 (42). Direct hospital costs and amortization costs of robotic and laparoscopic equipment were compared, assuming 300 and 500 cases per year, respectively. The robotic approach had higher mean costs than laparoscopic (robotic: $8,939; laparoscopic: $7,002) due to a 72% higher per-case amortization cost ($1,427 compared to only $42 for laparoscopic instruments). The authors concluded that the higher costs of amortization and disposable robotic instrumentation could not be justified given equivalent operative times and hospital stay.
Buzad and colleagues compared robotic vs. laparoscopic cholecystectomies at a single institution and analyzed operating room times, case volume, and instrument costs excluding amortization of the robot, utilities, and yearly maintenance (19). While operative times and instrument costs were similar ($1,325 versus $1,145), the robotic technique allowed that facility to perform a higher number of cholecystectomies per operative day. While no conclusions were drawn regarding total hospital costs, the authors concluded that instrument costs were similar between the two approaches, and that a RC program was more efficient overall than a laparoscopic program. Whether this conclusion can be justified by daily experience in the average operating room remains an open question.
A large population-based study conducted by Kamiński et al. examined differences in surgical outcomes and cost between robotic and laparoscopic cholecystectomies using 2010 and 2011 data from the National Inpatient Sample Health Cost Utilization Project (43). While length of stay and complication rates were similar, total hospital costs in the robotic group ranged from $4,333 to $8,310 higher than the laparoscopic group, despite excluding amortization costs for the robotic system.
Rosemurgy et al. analyzed costs associated with robotic and laparoscopic cholecystectomy at a single institution from the viewpoint of a hospital stakeholder (44). Amortization costs were excluded because purchasing the robot was allocated across the entire surgery department. While hospital costs for the robotic ($4,803) and laparoscopic ($4,655) cases were no different, patients were charged $8,000 more for RC ($33,801 versus $25,000). The higher charge was justified by the costs of the purchasing and maintenance contracts, specialized instruments, and specific team training. The authors concluded that hospitals passed along the costs associated with RC to consumers in the form of higher prices and were at least partially protected from higher robot-related expenses.
Similarly, Bedeir et al. evaluated 177 robotic and 281 laparoscopic cholecystectomies from 2012 to 2014 (45). The median total costs for RC were significantly lower than the laparoscopic technique (robot: $1,319; laparoscopic: $1,710). Savings were attributed to cheaper supplies, and to a lesser extent, shorter operating room times. However, amortization costs of the robot were excluded.
Finally, Schwaitzberg conducted a top-down analysis of robot versus laparoscopic cholecystectomy using data from Intuitive Surgical’s 2014 Investor Presentation and 2013 Investor Report (46). The accounting evaluated capital revenues ($835 million), service revenues ($397 million), and instrument revenues ($1,033 million) divided by the total number of robotic cholecystectomies performed divided by the number of installed robotic systems. Best and worst-case cost scenarios were calculated by adjusting for the model of robot purchased and the total number of procedures performed annually. The mean cost of a RC was $4,480, with best and worst-case scenarios of $2,908 and $8,675, respectively. Using similar techniques, the average instrument cost per case was $1,975 for robotic and $398 for laparoscopic procedure. Even in the best-case scenario, RC added approximately $2,600 to hospital costs per cholecystectomy.
Analysis of RC
Six studies evaluated costs of robotic compared to laparoscopic cholecystectomy. Four analyzed direct hospital costs (42-45) while one measured top-down costs (46) and one measured instrument costs (19). Two of the four hospital cost analyses demonstrated increased hospital costs of at least $1,900 (42,43). The top down analysis found a minimum additional cost of $2,600 per procedure (43) while the instrument price examination found equal instrument costs (19). Two studies found no significant difference (19,44) or minor savings of $391 dollars (45).
Published data indicate that overall costs of RC are declining over time [Breitenstein (42) 2008; Kamiński (43) 2014; Bedeir (45) 2016]. This may be due to institutional optimization (i.e., improvements caused by learning curve) and/or falling supplier costs from Intuitive Surgical Inc. Indeed, current data provided by Intuitive Surgical indicates the average cost of instrumentation and accessories for multi-port and single-site cholecystectomy, including the amortization of the robot, are now $1,251 and $863, respectively. These numbers are lower than cost data reported in prior studies, the reasons are unclear. Published data is difficult to compare interpret because of inconsistent analytic methods, variable inclusion of amortization and maintenance contract costs, and inability to track supply prices over time. Collectively, the data suggest no anticipated reduction in hospital stay after RC compared to laparoscopic. We conclude that hospitals are unlikely to derive financial or operational benefits from switching to RC.
Robotic HPB surgery
Existing cost accounting data associated with robotic HPB surgery is at best incomplete and unlikely to reflect the state of this field in the future. Current data comingle the learning curves for new surgical procedures being undertaken by HPB surgeons with costs derived from a market dominated by a single supplier of robotic instruments having a business plan to maximize its own revenue. Thus, the value proposition for stakeholders in this process cannot be defined.
The goal of robotic surgery is to convert open procedures into safe and effective minimally-invasive operations. Potential cost savings will therefore require reductions in inpatient hospital days compared to open as well as prevention of conversion events and associated complications. Existing data do not indicate that robotic surgery offers uniform benefits across the spectrum of HPB surgery. This is unsurprising as the major technical benefit of the robot is in suturing and fine dissection. A full accounting of the value equations also requires an analysis of the hidden costs required to achieve proficiency for surgeons and hospitals starting their learning curves for this complex technology. A prospective registry along the lines of an expanded ACS-NSQIP program that accounts for case volume and experience is a necessary step to expedite HPB surgery’s evolution into a minimal access specialty with a focus on patient quality of life. The merits of surgical procedures must be evaluated on an intent-to-treat basis, and the CPT coding and reimbursement process must be modernized to permit the value of surgical innovation to be passed along to the adopters of technologies with superior outcomes to open.
The coming renaissance
Any fair accounting of the current costs of robotic HPB surgery must acknowledge current market forces. A 2015 report published by the ECRI Institute, a consulting group for hospitals considering robotic surgery, labels ISI’s market dominance a “monopoly” (6). Fuller reached a similar conclusion regarding the high costs of robotic surgery and attributed the major driver to computer-enforced obsolescence of ISI Endowrist instruments (47). The ISI business model is reflected in the company’s earnings; 46% ($1,033 millions) of Intuitive’s $2.27 billion annual revenue is derived from instrumentation versus $835 million for robot purchasing and $397 million for maintenance (46). As a result, economic projections about future robotic surgery cannot be based on studies of prior costs. Rather, projections must incorporate the anticipated major impact of additional equipment manufacturers emerging in the market soon.
That process of freeing the marketplace may already be underway. Future market entrants are likely to launch a new age of expansion in robotic HPB surgery which will engineer a break from past market trends. Competition will increase the purchasing power of hospitals and drive innovation. We obtained previously unpublished cost data from Intuitive Surgical Inc. for this report. Current average cost for robotic instruments and accessories used during major pancreatic resection adds $2,007 to operative costs, an estimate significantly lower than $3,330 as reported by Butturini et al. (31). Further, a link between cost and case experience has been observed consistent with a learning curve effect; robotic surgery costs decreased 14.6% between 2011 ($23,595) and 2010 ($19,528) while laparoscopic costs remained stable ($15,286 and $15,195) (43).
Conclusions
All eighteen studies evaluating the economics of robotic HPB surgery have significant limitations. Not one study merged the interests of all involved stakeholders to construct a complete value proposition for HPB surgery. Many studies exclude amortization costs due to the inherent complexity of cost analysis or acknowledge conflicts of interest with Intuitive Surgical Inc. Little attention is devoted to the values patients care about. Future studies must incorporate (I) quality of life, survival, and return to independent function alongside data such as (II) intent-to-treat analysis of minimally-invasive surgery accounting for conversions to open, (III) surgeon and institution experience and operative time as surrogates for the learning curve; and (IV) amortization and maintenance costs as well as direct costs of disposables and instruments.
Acknowledgements
This work was supported by The Alliance of Families Fighting Pancreatic Cancer, the Greg and Cathy Griffith Family Foundation, and the John F. Fortney Charitable Pancreatic Cancer Research Group.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Porter ME. What is value in health care? N Engl J Med 2010;363:2477-81. [Crossref] [PubMed]
- Institute of Medicine (US) Roundtable on Value & Science-Driven Health Care, Yong PL, Olsen LA, et al. editors. Value in Health Care: Accounting for Cost, Quality, Safety, Outcomes, and Innovation. Washington (DC): National Academies Press (US), 2010.
- Samadi D. History of Robotic Surgery and FDA Approval. 2016. Available online: http://www.roboticoncology.com/
- Surgical Robots, Robotic Surgery, Robotic Systems. 2016. Available online: http://allaboutroboticsurgery.com/
- Feroldi B, Williams S, Swanson C. Where Will Intuitive Surgical Be in 10 Years? Available online: http://www.fool.com/investing/2016/05/31/where-will-intuitive-surgical-be-in-10-years.aspx
- ECRI Institute. Robotic Surgery. Available online: www.ecri.org/robotinfo
- Alpha Deal Group LLC. Could Titan Medical Storm the Robotic Surgery Market? 2014. Available online: http://lipperalpha.financial.thomsonreuters.com/2014/03/titan-storm-robotic-surgery-market/
- van der Schatte Olivier RH, Van't Hullenaar CD, Ruurda JP, et al. Ergonomics, user comfort, and performance in standard and robot-assisted laparoscopic surgery. Surg Endosc 2009;23:1365-71. [Crossref] [PubMed]
- Baker EH, Ross SW, Seshadri R, et al. Robotic pancreaticoduodenectomy for pancreatic adenocarcinoma: role in 2014 and beyond. J Gastrointest Oncol 2015;6:396-405. [PubMed]
- Boggi U, Amorese G, Vistoli F, et al. Laparoscopic pancreaticoduodenectomy: a systematic literature review. Surg Endosc 2015;29:9-23. [Crossref] [PubMed]
- Zureikat AH, Postlewait LM, Liu Y, et al. A Multi-institutional Comparison of Perioperative Outcomes of Robotic and Open Pancreaticoduodenectomy. Ann Surg 2016;264:640-9. [Crossref] [PubMed]
- Magge D, Gooding W, Choudry H, et al. Comparative effectiveness of minimally invasive and open distal pancreatectomy for ductal adenocarcinoma. JAMA Surg 2013;148:525-31. [Crossref] [PubMed]
- Hogg ME, Zenati M, Novak S, et al. Grading of Surgeon Technical Performance Predicts Postoperative Pancreatic Fistula for Pancreaticoduodenectomy Independent of Patient-related Variables. Ann Surg 2016;264:482-91. [Crossref] [PubMed]
- Surgical Instruments. FY2000 SEC. Available online: https://www.sec.gov/Archives/edgar/data/1035267/000109581101001899/f70521e10-k405.txt
- Srikrishna S, Robinson D, Cardozo L, et al. Is there a discrepancy between patient and physician quality of life assessment? Neurourol Urodyn 2009;28:179-82. [Crossref] [PubMed]
- Glickman SW, Boulding W, Manary M, et al. Patient satisfaction and its relationship with clinical quality and inpatient mortality in acute myocardial infarction. Circ Cardiovasc Qual Outcomes 2010;3:188-95. [Crossref] [PubMed]
- Shakir M, Boone BA, Polanco PM, et al. The learning curve for robotic distal pancreatectomy: an analysis of outcomes of the first 100 consecutive cases at a high-volume pancreatic centre. HPB (Oxford) 2015;17:580-6. [Crossref] [PubMed]
- Pepper D. 5 huge reputation management mistakes for surgeons. Available online: beckersspine.com
- Buzad FA, Corne LM, Brown TC, et al. Single-site robotic cholecystectomy: efficiency and cost analysis. Int J Med Robot 2013;9:365-70. [Crossref] [PubMed]
- Vidovszky TJ, Smith W, Ghosh J, et al. Robotic cholecystectomy: learning curve, advantages, and limitations. J Surg Res 2006;136:172-8. [Crossref] [PubMed]
- Jensen CC, Madoff RD. Value of robotic colorectal surgery. Br J Surg 2016;103:12-3. [Crossref] [PubMed]
- Chau Z, West JK, Zhou Z, et al. Rankings versus reality in pancreatic cancer surgery: a real-world comparison. HPB (Oxford) 2014;16:528-33. [Crossref] [PubMed]
- Falvo T, Grove L, Stachura R, et al. The opportunity loss of boarding admitted patients in the emergency department. Acad Emerg Med 2007;14:332-7. [Crossref] [PubMed]
- Tsung A, Geller DA, Sukato DC, et al. Robotic versus laparoscopic hepatectomy: a matched comparison. Ann Surg 2014;259:549-55. [Crossref] [PubMed]
- Daouadi M, Zureikat AH, Zenati MS, et al. Robot-assisted minimally invasive distal pancreatectomy is superior to the laparoscopic technique. Ann Surg 2013;257:128-32. [Crossref] [PubMed]
- Alasari S, Min BS. Robotic colorectal surgery: a systematic review. ISRN Surg 2012;2012:293894. [Crossref] [PubMed]
- Labor USDo. Employee Retirement Income Security Act (ERISA). Available online: https://www.dol.gov/agencies/ebsa/laws-and-regulations/laws/erisa
- Waters JA, Canal DF, Wiebke EA, et al. Robotic distal pancreatectomy: cost effective? Surgery 2010;148:814-23. [Crossref] [PubMed]
- Kang CM, Kim DH, Lee WJ, et al. Conventional laparoscopic and robot-assisted spleen-preserving pancreatectomy: does da Vinci have clinical advantages? Surg Endosc 2011;25:2004-9. [Crossref] [PubMed]
- Wayne M, Steele J, Iskandar M, et al. Robotic pancreatic surgery is no substitute for experience and clinical judgment: an initial experience and literature review. World J Surg Oncol 2013;11:160. [Crossref] [PubMed]
- Butturini G, Damoli I, Crepaz L, et al. A prospective non-randomised single-center study comparing laparoscopic and robotic distal pancreatectomy. Surg Endosc 2015;29:3163-70. [Crossref] [PubMed]
- Klompmaker S, van Zoggel D, Watkins AA, et al. Nationwide Evaluation of Patient Selection for Minimally Invasive Distal Pancreatectomy Using American College of Surgeons' National Quality Improvement Program. Ann Surg 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Baker EH, Ross SW, Seshadri R, et al. Robotic pancreaticoduodenectomy: comparison of complications and cost to the open approach. Int J Med Robot 2016;12:554-60. [Crossref] [PubMed]
- Boggi U, Signori S, De Lio N, et al. Feasibility of robotic pancreaticoduodenectomy. Br J Surg 2013;100:917-25. [Crossref] [PubMed]
- Ellison A. Average cost per inpatient day across 50 states. Available online: http://www.beckershospitalreview.com/finance/average-cost-per-inpatient-day-across-50-states-2016.html
- Scott DR; Center for Disease Control and Prevention. The direct medical costs of healthcare-associated infections in US hospitals and the benefits of prevention. Available online: http://www.cdc.gov/HAI/pdfs/hai/Scott_CostPaper.pdf, accessed September 1, 2012.
- Sham JG, Richards MK, Seo YD, et al. Efficacy and cost of robotic hepatectomy: is the robot cost-prohibitive? J Robot Surg 2016;10:307-13. [Crossref] [PubMed]
- Yu YD, Kim KH, Jung DH, et al. Robotic versus laparoscopic liver resection: a comparative study from a single center. Langenbecks Arch Surg 2014;399:1039-45. [Crossref] [PubMed]
- Packiam V, Bartlett DL, Tohme S, et al. Minimally invasive liver resection: robotic versus laparoscopic left lateral sectionectomy. J Gastrointest Surg 2012;16:2233-8. [Crossref] [PubMed]
- Ji WB, Wang HG, Zhao ZM, et al. Robotic-assisted laparoscopic anatomic hepatectomy in China: initial experience. Ann Surg 2011;253:342-8. [Crossref] [PubMed]
- Berber E, Akyildiz HY, Aucejo F, et al. Robotic versus laparoscopic resection of liver tumours. HPB (Oxford) 2010;12:583-6. [Crossref] [PubMed]
- Breitenstein S, Nocito A, Puhan M, et al. Robotic-assisted versus laparoscopic cholecystectomy: outcome and cost analyses of a case-matched control study. Ann Surg 2008;247:987-93. [Crossref] [PubMed]
- Kamiński JP, Bueltmann KW, Rudnicki M. Robotic versus laparoscopic cholecystectomy inpatient analysis: does the end justify the means? J Gastrointest Surg 2014;18:2116-22. [Crossref] [PubMed]
- Rosemurgy A, Ryan C, Klein R, et al. Does the cost of robotic cholecystectomy translate to a financial burden? Surg Endosc 2015;29:2115-20. [Crossref] [PubMed]
- Bedeir K, Mann A, Youssef Y. Robotic single-site versus laparoscopic cholecystectomy: Which is cheaper? A cost report and analysis. Surg Endosc 2016;30:267-72. [Crossref] [PubMed]
- Schwaitzberg SD. Financial modeling of current surgical robotic system in outpatient laparoscopic cholecystectomy: how should we think about the expense? Surg Endosc 2016;30:2082-5. [Crossref] [PubMed]
- Ness FJ. Surgical Technology. St. Louis, MO: Elsevier Sounders, 2005.


