Changes in dietary patterns and body composition within 12 months of liver transplantation
Introduction
Liver transplantation (LT) restores people’s lives; however, cardiometabolic risk factors are increasingly impacting on survival (1), with a higher prevalence of cardiovascular events in liver transplant recipients (LTR) compared to the general population (2). These risk factors include obesity, new onset diabetes and the metabolic syndrome (1,3). In addition, obesity increases hepatic steatosis and recurrence or de novo non-alcoholic fatty liver disease (NAFLD). This may contribute to the severity of other recurrent diseases such as hepatitis C (4-6). Early identification, prevention and management of post-transplant obesity may positively impact on these liver related issues and the development of co-morbidities, which in turn may impact long-term post-transplant survival (7).
Obesity in adult post-LTRs appears to be a world-wide phenomenon (7) and affects more than 20% of patients in the first year after transplantation (8-10). Weight gains of approximately 10% dry body weight are observed at 6 months post-transplant, and gradually increase to ~20% by 3 years (9). However, there is little data as to what proportion is due to replenishment of muscle mass, or the accumulation of fat stores (11).
There is also a paucity of data regarding the changes in dietary intake and habits post-transplant, for example food group intake and snacking and in particular the impact of psychological factors such as recovering from a period of significant illness coupled with intrusive dietary restriction; and surviving a life-threatening disease which may influence eating behaviour.
There are limited guidelines to inform diet and exercise advice beyond the immediate period post liver transplant, whether these are general recommendations, specific to the obese patient; or for the management of recurrent disease such as hepatic steatosis. The American Society of Transplantation and the United Network for Organ Sharing provide some lifestyle recommendations, albeit brief and generic, for hypertension and diabetes management post solid organ transplant (12,13).
With these issues in mind, the aim of the study was to prospectively assess changes in weight, nutritional status, body composition, dietary intake and psychological characteristics including perceived stress and depressive symptoms in patients before and at 6 and 12 months post liver transplant.
Methods
This single-centre prospective observational cohort study was commenced in March 2012, at the Princess Alexandra Hospital (PAH), within the Queensland Liver Transplant Service. Ethical approval was obtained from the PAH Human Research Ethics Committee (approval number HREC/12/QPAH/81-SSA/12/QPAH/97). Informed consent in writing was obtained from the each patient.
Subject recruitment
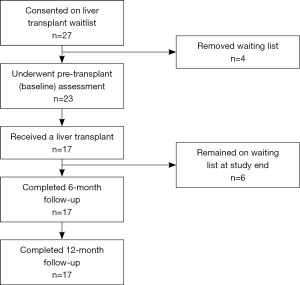
Patients awaiting liver transplant were invited to participate in the study during the recruitment phase from March 2012 to June 2013. Eleven patients received a transplant prior to being approached for inclusion in the study and were not included in the observations. All patients who were approached to participate following active listing (n=27) consented to the study but only those transplated by December 2014 (n=17) were included in the 6 and 12 month post-transplant assessment (see Figure 1).
Medical history
Patient medical history including age, gender and peri-operative complications, aetiology of liver disease and Model for End-stage Liver Disease (MELD) score were collected from the medical chart and the hospital liver transplant database and the primary indication for liver transplant was assessed by a consultant gastroenterologist.
Anthropometry and nutritional status
Weight and waist circumference were measured at 6 and 12 months post-transplant. Due to the presence of ascites in some patients pre-transplant, a dry weight measured at one month post-transplant was used as baseline weight for all patients to calculate baseline body mass index (BMI) and weight change by 6 and 12 months post-transplant. Percentage body fat was assessed using bioelectrical spectroscopy (ImpediMed SBF7, Impedimed, Australia; normal range males 8% to 24%, females 21% to 35%) (14).
Subjective global assessment (SGA) (15) was used to assess nutritional status at pre-transplant and at 6 and 12 months. Hand grip strength (HGS) was also assessed at the three time points as a mean of both hands using an electronic dynamometer (Camry model: EH101; normal HGS males ≥30 kg and females ≥20 kg) (16). An oral glucose tolerance test was performed by Queensland Health Pathology at 6 months, as part of standard clinical practice at this centre.
Physical activity
Physical activity was assessed pre-transplant and 6 months post-OLT using the International Physical Activity Questionnaire-Short Form (17). The questionnaire incorporates seven items to assess varying intensities of physical activity that include walking, moderate physical activity, vigorous physical activity, and sitting, reported as metabolic equivalent task score per week (METS-minutes/week).
Dietary assessment
Usual dietary intake data were collected via an in-depth diet history, where the participants’ self-recorded usual intake of food and beverages consumed over a seven day period. The record was validated by an interview with an accredited practicing dietitian, which involved quantification of portion sizes and where possible, capturing brand names. Dietary data were collected pre-transplant, and at 6 and 12 months post-transplant by the same research dietitian. These data were analysed using FoodWorks 7® Professional (Xyris Software, Australia) using the NUTTAB 2010 database to determine average daily energy, protein, and sodium intakes and percentage energy contribution from macronutrients (carbohydrate, fat, protein). Energy and protein requirements were calculated for each study participant based on the ESPEN guidelines for nutrition in liver disease and transplantation (18). Pre-transplant requirements were calculated as 147–168 kJ/kg of adjusted body weight/day and 1.2–1.5 g protein/kg of adjusted body weight/day. The respective energy and protein requirements post-transplant were 100–125 kJ/kg of adjusted body weight/day and 0.8–1.0 g/kg of adjusted body weight/day (19). For overweight or obese patients an adjusted body weight was used for all calculations [adjusted body weight = (ideal body weight at BMI 25 kg/m2 ×0.025) + ideal body weight at BMI 25 kg/m2] and reported as kJ/kg of adjusted body weight and g/kg of adjusted body weight. Participants were considered energy under-reporters if the energy intake to basal metabolic rate ratio was below 0.9 (20).
Food group analysis was performed for the diets of each participant using the latest Australian food-nutrient databases, AUSNUT 2011–2013 (21). In this database each food is allocated a unique code, which is used to group foods into 24 major food groups. Food intake of the study participants was compared with the national medians as reported in National Nutrition and Physical Activity Survey, Australian Health Survey (AHS) (22).
Psychological factors
Responsiveness to the food environment was assessed pre-transplant and at 6 months post-transplant using the validated 15-item Power of Food Scale (23,24). Patients answered on a 5-point Likert scale (ranging from 1= don’t agree, to 5= strongly agree), to assess the psychological impact of a food-abundant environment, with a higher score indicating a stronger appetitive drive. Perceived stress, depressive symptoms, self-esteem levels, and disordered food habits such as night snacking were assessed using the Weight and Lifestyle Inventory (WALI) (25).
Statistical analyses
Participant characteristics are reported as mean ± standard deviation or median [interquartile range (IQR)] depending on the distribution of the data. Repeated measures ANOVA (continuous variables), with post-hoc Tukey’s test, or Friedman Test, and Cochran’s Q (categorical variables) were used to identify differences in variable between the three time points. Paired t-test or Wilcoxon Signed rank test were used for continuous and categorical variables, respectively, when data from only two time points were available. Comparison of food group intake with the Australian population and between 6 months post-transplant and 12 months post-transplant were assessed using Mann-Whitney Test and Wilcoxon Signed Rank Test, respectively. Spearman’s rho was used to assess the association between HGS and percentage body fat mass. Statistical significance was reached when P<0.05 and all statistical analyses were conducted in Statistical Package for the Social Sciences (SPSS®) Version 22 (IBM Corporation). Cases with missing data were excluded from their related analyses.
Results
Pre-transplant characteristics
Figure 1 outlines the number of participants invited and recruited in this prospective study. Of the 27 participants consented to the study, four were removed from the liver transplant list prior to pre-transplant assessment (n=3 for disease progression and n=1 due to improvement in liver function). Twenty-three participants [6 women (median age, 49 years) and 17 men (median age, 53 years)] underwent pre-transplant assessment; however six were still awaiting LT when recruitment ended. Seventeen orthotopic liver transplantations (OLT) were performed [median age 54 years (IQR 16 years), males n=14], with a median waiting time of 12 months (range, 4 to 30 months) between listing and transplant. The study cohort was representative of all patients active on the waiting list between 2011 and 2012 in age (P=0.48), gender (P=0.76) and MELD score (P=0.97).
The median MELD score was 15. Six patients had a MELD score of less than 15. Three of these were suffering recurrent bouts of cholangitis requiring intravenous antibiotics and hospital admission, two with previous Kasai procedures as neonates and a third with PSC (MELDs 6, 7 and 13 respectively). One patient had suffered a hepatic artery thrombosis after previous liver transplant for chronic HBV and now had recurrent sepsis (MELD 12). A fifth patient had high output cardiac failure related to hereditary haemorrhagic telangiectasia with the vascular abnormalities largely confined to the liver (MELD 8); while the final patient had liver disease from HCV and alcohol with intractable hepatic encephalopathy (MELD 13). Three patients had small HCCs, only one with a diameter >2.0 cm, and their calculated MELDs were 15, 16 and 17.
A standard triple therapy calcineurin inhibitor immunosuppression regime was employed, with steroid induction. Post-OLT all participants went on tacrolimus, azathioprine, and prednisolone, with the azathioprine and prednisolone generally weaned after 3 months (only one patient remained on prednisolone by 6 months post-OLT). Serum tacrolimus levels were maintained at an average of 8.2 µg/L (range, 5.5–16.6 µg/L) at 6 months post-transplant and 6.6 µg/L (range, 3.0–9.9 µg/L) at 12 months post-transplant. Six participants had peri-operative complications (such as rejection and infection) but did not differ in age, gender or BMI from those without such complications.
Change in nutritional status
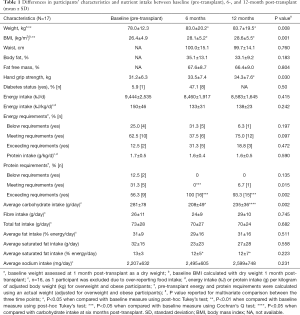
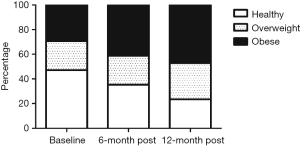
Mean weight at the three time points is presented in Table 1 and BMI change over 12 months is illustrated in Figure 2. Weight change was variable, but on average participants gained 7.3% (95% CI, 2.2% to 14.2%) of body weight by 12 months post-OLT with the majority of the weight gain [6.4% (95% CI, 1.7% to 12.2%)] occurring within 6 months of transplant. This led to an increase in those classified as overweight or obese, from a baseline of 53% (n=9/17) to 77% (n=13/17) by 12 months-post OLT (Figure 2). Eight participants (47%) were malnourished (SGA-B) pre-transplant. This resolved within 6 months of transplant, with all patients classified as well-nourished (SGA-A) through to 12 months post-OLT. Handgrip strength was 32.9±5.4 kg in males and 22.1±2.5 kg in females pre-transplant and was not correlated with age (P=0.31). Reduced muscular strength, [as classified by HGS <30 kg males and <20 kg females (16)], was present in 4 patients pre-transplant and this persisted at 12 months in 3 patients despite these individuals experiencing weight gain ranging from 6.5 to 23.3 kg and being overweight or obese at 12 months. There was a negative correlation between HGS and body fat percentage (r=−0.67, P=0.012). There was no relationship between initial BMI and amount of weight gain (P=0.96).
Full table
There was no change in overall median physical activity after transplant [790 MET-minute/week (IQR 243−2,098 MET-minute/week) vs. 956 MET-minute/week (IQR 604−6,021 MET-minute/week), P=0.124]. However, within the categories of exercise intensity, there was a 50% increase in walking activity reported by 6 months post-transplant [429 MET-minutes/week (IQR 50−644 MET-minutes/week) vs. 644 MET-minutes/week (IQR 446−2,079 MET-minutes/week), P=0.028] and no change in moderate or vigorous activities.
Oral glucose tolerance test at 6 months post-OLT revealed 65% (n=11/17) of patients had altered glucose metabolism (new onset diabetes n=7; persistent pre-existing diabetes n=1; impaired glucose tolerance n=3).
Dietary characteristics
One participant was excluded from the dietary analyses due to under-reporting, defined by the Goldberg cut-off (20). Table 1 shows nutrient intake at the three time points and the proportion of patients below, meeting, or exceeding their estimated energy needs pre- and post-OLT. At 6 months post-OLT there was substantial variation in those below, meeting and exceeding energy requirements. This appeared to resolve by 12 months post-transplant with the majority meeting their energy needs. Once transplanted, no significant changes were observed in protein intake over a period of 12 months. Despite a measurable increase in carbohydrate intake between 6 and 12 months post-OLT, intake of carbohydrates remained significantly lower than that recorded pre-transplant. Sodium intake remained that of a ‘no added salt’ diet (i.e., <100 mmol/day).
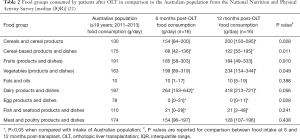
Specific food group analysis indicated that during the course of transplant recovery, the intake of carbohydrate-rich food groups such as ‘Cereal-based products and dishes’ (including sweet pastries, cakes, and pies), ‘Cereal products and dishes’ (such as breads, pasta, breakfast cereals), and ‘Vegetables’ (including sweet corn and potatoes) increased (Table 2). Compared to the general Australian population, LTRs consumed less refined carbohydrates, less fish/seafood, and more vegetables and dairy products (Table 2). There was a sustained avoidance of eggs during the whole 12 months post-OLT period. The intake of sugar sweetened beverages and sugar products and dishes post transplant was also low.
Full table
Psychological factors
The impact of behavioural and emotional factors typically associated with excess weight such as stress levels, presence of depressive symptoms, self esteem and appetitive drive associated with food experience and dietary behaviours was assessed by questionnaire. At 6 months post-OLT, more than half the patients (56%, n=9/16) perceived low to average levels of stress during the last 6 months and most (81%, n=13/16) anticipated similar levels of stress in the next 6 months. Over half of the patients (69%, n=11/16) reported low to no depressive feelings and rated their self-esteem as good (53%, n=9/16) or average (31%, n=5/16). Night snacking, i.e., waking up from sleep to eat, was present in 75% of patients’ pre-transplant and only 25% 6 months post-OLT. The Power of Food Scale increased from pre-transplant to 6 months after transplant, which was comparable to that of an obese population with diabetes (22), indicating a stronger appetitive drive (1.6±0.5 vs. 1.9±0.4, P=0.028).
Discussion
This descriptive longitudinal study identified significant weight gain (6.4% of body weight on average) in the first six months following LT. By 12 months post-OLT, patients had gained on average 7.3% of body weight. Furthermore, two-thirds of transplant recipients had evidence of impaired glucose metabolism by 6 months post-OLT. Replenishing muscle stores is an anticipated (26), beneficial consequence of liver transplant, and there was evidence of improved muscle function with improvement in HGS (mean 3.1 kg by 12 months post-transplant). However, by 6 months, two out of every three patients were centrally obese, indicating unhealthy fat deposition. Once present post-OLT, excess body fat persists long term (11). Thus the current study highlights the early onset of fat accumulation and implies there should be early steps to identify and address post-OLT obesity.
Retrospective studies have identified larger weight gains post-OLT (10–20% within the first year) (8-10). However, the weight gain in this study is in agreement with a prospective cohort study of 597 LTRs (5.1 kg in the first year post-OLT) (8).
Previous studies have examined weight gain in relation to risk factors such as age, aetiology of liver disease and type of immunosuppression (8,9,11). Corticosteroids have well-recognised effects on appetite and weight gain (12,13), however in our centre there is a strategy to try to wean prednisolone by three months and only one patient remained on this (at 5 mg/d) at six months.
The return of a normal diet and appetite have also been implicated as contributors to weight gain post-OLT (8,27), yet this is the first study to report on the dietary patterns and diet quality in this unique patient population, using robust prospective dietary assessment methods. When compared to the Australian adult population, LTRs had similar intakes of meat, fruit, and oils, and a high saturated fat intake, consistent with a Western-style dietary pattern, but lower intake of processed carbohydrate foods (cereal-based products and dishes). By 12 months, patients were consuming more vegetables (equivalent to one additional serve per day) and had approximately 40% higher intakes of dairy products compared to population norms. There appeared to be a sustained avoidance of eggs and fish post-transplant.
In general, patients with advanced liver disease awaiting transplant are prescribed a high protein, high energy, reduced sodium diet (28) to preserve muscle and fat stores and reduce fluid accumulation. To achieve this requires a high intake of dairy products and meats or meat alternatives. They are encouraged to eat frequent meals and have night-time snacks, evident in 75% of this sample pre-transplant (29).
Immuno-compromised patients, such as these in the acute OLT period, are considered at increased risk of food-borne infections (30). While there is a lack of reliable data regarding the rates of post-transplant food-borne illness, a conservative dietary approach is often taken for these patients (30,31). This may explain the low intake of eggs and seafood in our patients compared to the general Australian population. Other dietary patterns post-OLT also appear to reflect pre-transplant advice, and whilst patients mostly cease night snacking, they maintain high protein intakes and a sodium intake two thirds that of the general population (32).
Obese LTRs are at increased risk of late complications such as hepatic steatosis, type 2 diabetes and post-transplant malignancies (33). After cardiovascular complications, de novo malignancies are the second leading cause of death in transplant recipients (33). Diabetes and poor glycaemic control are associated with adverse outcomes post transplant (34). Effective prevention and management of post-OLT co-morbidities may improve patient survival and quality of life. Lifestyle intervention, including physical activity and dietary pattern modification may mitigate risk of these post-OLT complications.
The Western-style dietary pattern observed may exacerbate these metabolic risk factors in the setting of obesity. A shift towards a Mediterranean-style diet has been shown to be beneficial in cardiovascular health, glycemic control, reducing hepatic steatosis and insulin resistance (35-37). The Mediterranean dietary pattern promotes plant-based foods, healthy fats (with emphasis on olive oil and nuts), limiting red and processed meat and dairy products and consuming fish regularly (38). Further studies investigating the potential beneficial effects of targeting key aspects of the Mediterranean dietary pattern (decreasing red meat and dairy; and increasing intake of fish, nuts, vegetables and unsaturated oils) are of interest in the LTR population.
LTRs increased physical activity post-OLT, with a switch from predominantly sitting/sedentary activity pre-transplant to lightly active lifestyle post-transplant. This was not sufficient to ameliorate increased rates of metabolic dysfunction or increases in fat mass. The negative correlation between change in HGS and fat mass suggests those that are gaining the fattest are also gaining least muscle quality. A greater intensity of physical activity is probably required to have benefits on metabolic dysfunction (39), and an early focus on achieving this appears warranted. HGS has been inversely associated with cardiovascular disease and cardiovascular mortality in the general population (40) and may be a valuable addition to standard post-transplant monitoring protocols to predict those at risk of adverse cardiovascular health long term.
This study assessed a number of behaviours and emotional factors typically associated with obesity; and hedonic characteristics associated with food experience in an obesogenic food environment. All but one patient met or exceeded predicted energy intake requirements at 12 months post-OLT. There was an increase in the Power of Food score after transplant suggesting that appetitive drive increases early and by 6 months is similar to that of an obese diabetic population (24). In other obese patient populations in a tertiary hospital setting, the prevalence of depressive symptoms is as high as 65% (41). Despite the increasing prevalence of overweight and obesity, LTRs report emotional factors and behaviours that are in significant contrast to this. In general most of our cohort had minimal to no depressive feelings; with healthy ratings for self-esteem and minimal evidence of disordered eating behaviours. This is of clinical importance when designing lifestyle interventions for this patient population.
This study had a number of limitations. It was underpowered to undertake multivariate analysis to explore the independent drivers for the variability of weight change observed (note large SD/95% CI). The disease etiology and time since diagnosis may impact on the results of this study, however with the small data set a sub group analysis is not appropriate. Immunosuppressive agents such as prednisolone and tacrolimus may impact on metabolism, however due to the small data set conclusions cannot be drawn regarding the impact of individual immunosuppressive agents or doses. The observational nature of the study cannot determine causation between dietary intake (i.e., amount and pattern), weight change and metabolic outcomes. Longer term cardiometabolic outcomes were not captured, however the finding that weight gain slows after the first 6 months post-OLT is clear. The investigators invested considerable attention to validating patient recall of dietary intake and physical activity data, however inherent limitations remain with reliance on self-reported data collection. Higher quality methods of assessing body composition such as CT or MRI may have improved the precision of the body fat data and should be considered for future studies, however the use of low cost, accessible methodologies such as BIA assist with real world translation of practice change. Finally, this work did not explore key drivers in dietary pattern choices, therefore can only hypothesize about legacy effect of dietary recommendations pre- and immediately post OLT.
While weight gain after transplantation is well documented, there is a gap in evidence-based lifestyle intervention to prevent and mitigate cardiometabolic risk factors in the first year post-OLT. In the current study, we show that excess weight gain occurs early post-transplant; is predominantly due to gain in fat mass; and is observed in concert with developed metabolic disturbances such as impaired glucose tolerance and diabetes. Part of the post-transplant weight gain may relate to western style dietary patterns, continuation of pre-transplant food choices and low intensity physical activity. The emotional drivers and response to excess weight gain in OLT-recipients seem distinct from those seen in the general obese population. These data could inform strategies to address weight gain with the most obvious of these being that interventions to limit weight gain should occur early after LT and should be tailored to meet the characteristics of this unique patient population.
Acknowledgements
We would like to thank Nutrition and Dietetics Department, Princess Alexandra Hospital; Queensland Liver Transplant Service, Princess Alexandra Hospital.
Funding: This work was supported by the Dietitians Association of Australia (Small Grant Program 2011); and Princess Alexandra Hospital Research Foundation (Small Grant 2011).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Ethical approval was obtained from the PAH Human Research Ethics Committee (Approval number HREC/12/QPAH/81-SSA/12/QPAH/97). Informed consent in writing was obtained from each patient.
References
- Lv C, Zhang Y, Chen X, et al. New-onset diabetes after liver transplantation and its impact on complications and patient survival. J Diabetes 2015;7:881-90. [Crossref] [PubMed]
- Madhwal S, Atreja A, Albeldawdi M, et al. Is liver transplantation a risk factor for cardiovascular disease? A meta-analysis of observational studies. Liver Transpl 2012;18:1140-6. [Crossref] [PubMed]
- Pagadala M, Dasarathy S, Eghtesad B, et al. Posttransplant metabolic syndrome: An epidemic waiting to happen. Liver Transpl 2009;15:1662-70. [Crossref] [PubMed]
- Seo S, Maganti K, Khehra M, et al. De novo nonalcoholic fatty liver disease after liver transplantation. Liver Transpl 2007;13:844-47. [Crossref] [PubMed]
- Patil DT, Yerian LM. Evolution of nonalcoholic fatty liver disease recurrence after liver transplantation. Liver Transpl 2012;18:1147-53. [Crossref] [PubMed]
- Vallin M, Guillaud O, Boillot O, et al. Recurrent or de novo nonalcoholic fatty liver disease after liver transplantation: Natural history based on liver biopsy analysis. Liver Transpl 2014;20:1064-71. [Crossref] [PubMed]
- Watt KD, Charlton MR. Metabolic syndrome and liver transplantation: A review and guide to management. J Hepatol 2010;53:199-206. [Crossref] [PubMed]
- Richards J, Gunson B, Johnson J, et al. Weight gain and obesity after liver transplantation. Transpl Int 2005;18:461-66. [Crossref] [PubMed]
- Rezende Anastácio L, García Ferreira L, Costa Liboredo J, et al. Overweight, obesity and weight gain up to three years after liver transplantation. Nutr Hosp 2012;27:1351-56. [PubMed]
- Kouz J, Vincent C, Leong A, et al. Weight gain after orthotopic liver transplantation: Is nonalcoholic fatty liver disease cirrhosis a risk factor for greater weight gain? Liver Transpl 2014;20:1266-74. [Crossref] [PubMed]
- Anastácio LR, Ferreira LG, de Sena Ribeiro H, et al. Body composition and overweight of liver transplant recipients. Transplantation 2011;92:947-51. [Crossref] [PubMed]
- American Society of Transplantation. Health after transplantation. Available online: http://onlinelibrary.wiley.com/doi/10.1002/lt.23566/full. Access: 22 May 2015.
- United Network for Organ Sharing. Staying healthy: Diet & exercise. Available online: https://www.unos.org/wp-content/uploads/unos/WEPNTK.pdf. Access: 22 May 2015.
- Gallagher D, Heymsfield SB, Heo M, et al. Healthy percentage body fat ranges: An approach for developing guidelines based on body mass index. Am J Clin Nutr 2000;72:694-701. [PubMed]
- Bauer J, Capra S, Ferguson M. Use of the scored patient-generated subjective global assessment (pg-sga) as a nutrition assessment tool in patients with cancer. Eur J Clin Nutr 2002;56:779-85. [Crossref] [PubMed]
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the european working group on sarcopenia in older people. Age Ageing 2010;39:412-23. [Crossref] [PubMed]
- Bassett DR Jr. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003;35:1396. [Crossref] [PubMed]
- Plauth M, Merli M, Kondrup J, et al. Espen guidelines for nutrition in liver disease and transplantation. Clin Nutr 1997;16:43-55. [Crossref] [PubMed]
- National Institute for Health and Care Excellence: Nutrition support in adults: Oral nutrition support, enteral tube feeding and parenteral nutrition (cg32). NICE Guidelines, 2006.
- Goldberg GR, Black A, Jebb S, et al. Critical evaluation of energy intake data using fundamental principles of energy physiology: 1. Derivation of cut-off limits to identify under-recording. Eur J Clin Nutr 1991;45:569-81. [PubMed]
- Food Standards Australia New Zealand. Food nutrient database. Available online: http://www.foodstandards.gov.au/science/monitoringnutrients/ausnut/foodnutrient/Pages/default.aspx. Access: 29th October 2014.
- Australian Bureau of Statistics: Median amount of foods consumes (grams) (a): Major and sub-major food groups (b). In: Australian Health Survey: Nutrition first results - Foods and nutrients - cat. no. 43640DO006_20112012. Canberra: ABS, 2014. Available online: http://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/4364.0.55.0072011-12?OpenDocument
- Lowe MR, Butryn ML, Didie ER, et al. The power of food scale. A new measure of the psychological influence of the food environment. Appetite 2009;53:114-8. [Crossref] [PubMed]
- Cappelleri JC, Bushmakin AG, Gerber RA, et al. Evaluating the power of food scale in obese subjects and a general sample of individuals: Development and measurement properties. Int J Obes (Lond) 2009;33:913-22. [Crossref] [PubMed]
- Wadden TA, Foster GD. Weight and lifestyle inventory (wali). Obesity 2006;14:99S-118S. [Crossref] [PubMed]
- Bergerson JT, Lee JG, Furlan A, et al. Liver transplantation arrests and reverses muscle wasting. Clin Transplant 2015;29:216-21. [Crossref] [PubMed]
- Ribeiro HS, Anastácio LR, Ferreira LG, et al. Energy expenditure and balance among long term liver recipients. Clin Nutr 2014;33:1147-52. [Crossref] [PubMed]
- Plauth M, Cabre E, Riggio O, et al. Espen guidelines on enteral nutrition: Liver disease. Clin Nutr 2006;25:285-94. [Crossref] [PubMed]
- Tsiaousi ET, Hatzitolios AI, Trygonis SK, et al. Malnutrition in end stage liver disease: Recommendations and nutritional support. J Gastroenterol Hepatol 2008;23:527-33. [Crossref] [PubMed]
- McGeeney LM, Gatiss GA. A survey of food safety information and foodborne infections post solid organ transplant. e-SPEN Journal 2014;9:e195-9.
- Acheson D. Iatrogenic high-risk populations and foodborne disease. Infect Dis Clin North Am 2013;27:617-29. [Crossref] [PubMed]
- National Health and Medical Research Council. Nutrient reference values for Australia and New Zealand. ed. Canberra, ACT: Australian Government Department of Health and Ageing, 2006.
- Chak E, Saab S. Risk factors and incidence of de novo malignancy in liver transplant recipients: A systematic review. Liver Int 2010;30:1247-58. [Crossref] [PubMed]
- Morbitzer KA, Taber DJ, Pilch NA, et al. The impact of diabetes mellitus and glycemic control on clinical outcomes following liver transplant for hepatitis C. Clin Transplant 2014;28:862-8. [Crossref] [PubMed]
- Ryan MC, Itsiopoulos C, Thodis T, et al. The mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol 2013;59:138-43. [Crossref] [PubMed]
- Sleiman D, Al-Badri MR, Azar ST. Effect of mediterranean diet in diabetes control and cardiovascular risk modification: A systematic review. Front Public Health 2015;3:69. [Crossref] [PubMed]
- Turati F, Trichopoulos D, Polesel J, et al. Mediterranean diet and hepatocellular carcinoma. J Hepatol 2014;60:606-11. [Crossref] [PubMed]
- Willett WC, Sacks F, Trichopoulou A, et al. Mediterranean diet pyramid: A cultural model for healthy eating. Am J Clin Nutr 1995;61:1402S-06S. [PubMed]
- Yates T, Henson J, Edwardson C, et al. Objectively measured sedentary time and associations with insulin sensitivity: Importance of reallocating sedentary time to physical activity. Prev Med 2015;76:79-83. [Crossref] [PubMed]
- Leong DP, Teo KK, Rangarajan S, et al. Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet 2015;386:266-73. [Crossref] [PubMed]
- Hickman IJ, Davis AC, Whelan ME, et al. Depressive symptoms and obesity: Assessing and addressing the black dog in the room. Nutr Diet 2012;69:234-35. [Crossref]


