Management of colorectal cancer with synchronous liver metastases: impact of multidisciplinary case conference review
Introduction
Patients with synchronous colorectal cancer and colorectal liver metastases (CRLM) pose a unique oncological challenge due to the presence of two disease sites. A variety of treatment approaches exist, and consensus on the optimal sequence of management has not been reached (1). Optimal treatment requires increasingly specialized expertise and input from various disciplines. Each case requires a unique care plan dependent on patient, primary disease site, and metastatic burden, making treatment increasingly patient specific. Multidisciplinary case conference (MCC) review is encouraged in these complex cases as MCCs are thought to improve quality of care (2-9). While many of these cases undergo MCC review, the specific benefit of MCC presentation in colorectal cancer care is currently unknown. Such knowledge is essential to inform future recommendations regarding the presentation of patients with synchronous disease at MCC.
At presentation, 23–51% of patients diagnosed with colorectal cancer have synchronous liver metastases (10). Patients with synchronous disease require co-ordination of multiple providers in order to optimize the timing and sequence of therapies, with the management of rectal cancer and synchronous metastatic disease posing an especially difficult dilemma. In patients with colorectal cancer and synchronous liver metastases, surgical resection of all disease is the only curative option and achieves five-year survival in 23–39% of cases (1,11) compared to 2.9% in those treated with palliative chemotherapy alone (12). The conventional two-staged approach to surgical treatment involves colorectal surgery first, followed by delayed resection of liver metastases; however, some patients may benefit from a liver-first surgical approach delaying colorectal surgery until after recovery and possible chemotherapy (1). More recently, there has been greater enthusiasm for one-stage simultaneous resection of the primary and metastatic disease, minimising the potential for tumor progression and avoiding multiple major surgeries (13-15). Simultaneous resection simplifies adjuvant treatment planning by eliminating the induction period between operations during which disease progression could occur and the benefits of chemotherapy are not clear. However, concerns persist about the safety of simultaneous resection, particularly for patients with extensive liver metastases. Irrespective of surgical approach, chemotherapy and radiation play an integral role in the management of patients with synchronous metastatic disease. Perioperatively, administration of chemotherapy has been shown to improve progression-free survival following resection of CRLM (16) and the addition of neoadjuvant radiation in appropriate patients with rectal cancer reduces the rate of pelvic recurrence (17). These issues underscore the importance of MCC discussion and a carefully planned multidisciplinary approach in these patients.
MCCs are thought to improve care through a variety of mechanisms. The representation of multiple specialties enables expedient decision making, and discussion of complex cases amongst multiple providers is expected to generate more evidence-based recommendations. Furthermore, provider confidence and patient satisfaction may be positively impacted due to the perception of higher quality multi-disciplinary care. As such, MCCs have risen in prominence and are now widespread amongst cancer programs (18). Remarkably, no studies have focused on patients with synchronous primary colorectal cancer and resectable metastatic disease. These patients, with potentially curable disease at two sites, pose a unique challenge and stand to benefit greatly from MCC discussion. Co-ordination amongst colorectal surgeons, hepatobiliary surgeons, medical and radiation oncologists, radiologists, and pathologists may expedite decision making and improve adherence to evidence-based guidelines. This study was conducted in order to determine the access to and association between MCC review and management amongst patients with colorectal cancer and synchronous liver metastases.
Methods
Study design
A retrospective cohort study was conducted in order to determine the association between MCC participation and the process of care for patients with synchronous colorectal cancer and liver metastases. The study was approved by the Western University Office of Research Ethics and the Lawson Health Research Institute (REB # 106917).
Patients and data
Data on all patients with a diagnosis of synchronous colorectal cancer and liver metastases who underwent elective liver resection with curative intent at London Health Sciences Centre (LHSC) between January 2008 and June 2015 were collected. LHSC is a tertiary care academic institution in London, Ontario, Canada with a catchment area of approximately 1.6 million people. Cancer Care Ontario has mandated that over 90% of all hepato-pancreatic-biliary (HPB) cancer surgeries in the province of Ontario take place at designated HPB centres, and LHSC is the only designated HPB centre inside its catchment area. A prospectively maintained database of patients undergoing liver resection at LHSC was used to identify eligible patients and additional data were retrospectively collected from both the database and the patients’ medical record. Synchronous disease was defined as CRLM identified at or before surgery for the primary tumor, in agreement with international multidisciplinary consensus (1). In this cohort, all patients had suspicious or confirmed metastatic liver lesions present on pre-operative imaging. Liver resection was classified into minor (<4 segments) or major (≥4 segments) (19). Patients who underwent resection of their colorectal primary tumor on an emergent basis for bleeding, obstruction, or perforation were excluded from the cohort on the basis of information available in the complete medical record.
MCC
A weekly multidisciplinary gastrointestinal cancer conference has been in operation at LHSC since January 2008. Colorectal cancer represents the largest volume of cases discussed. Cases are submitted on a voluntary basis. Physicians from LHSC and nine community hospitals in the surrounding region, for which LHSC serves as the tertiary referral center, are invited to present patients. The MCC is attended by general surgeons, colorectal surgeons, surgical oncologists, hepatobiliary surgeons, medical oncologists, radiation oncologists, pathologists, and diagnostic radiologists as mandated by Cancer Care Ontario (20). Videoconferencing connects all participating hospitals with real-time interaction. Following discussion, the consensus recommendation of the MCC is documented in the patient’s electronic medical record. Conventional, liver-first, and simultaneous approaches are all considered, along with timing of chemotherapy and radiation.
Variables and analysis
Data on patient demographics, pre-operative staging, MCC recommendations, operative details, and post-operative outcomes were collected. Height and weight data was missing for 5 (8%) patients. These patients were excluded from analyses involving height, weight, or BMI. All other data was available for all patients. Univariate analysis was used to assess whether any variables influenced presentation at MCC, and whether treatment decisions were impacted by MCC recommendations. Continuous variables were expressed as medians (interquartile range) and were compared using the Mann-Whitney-U test. Categorical variables were compared using the Fisher’s exact test. P<0.050 was considered statistically significant. All statistical analysis was performed using IBM SPSS Statistics for Windows, version 23 (IBM Corp., Armonk, NY, USA).
Results
Sixty-six patients underwent liver resection with curative intent for synchronous colorectal cancer with liver metastases in the seven-year study period. Twenty-nine (44%) cases were presented at the gastrointestinal MCC for discussion. Of these, the majority (25 of 29, 86%) were presented by a tertiary care provider from LHSC. The remaining 4 (14%) cases were presented via videoconferencing by medical oncologists in community hospitals. No cases were presented by a surgeon from a community hospital. Of the 37 patients who were not presented at MCC, 43% (16 of 37) underwent elective resection of their primary tumor in a community hospital prior to consultation with a hepatobiliary surgeon at LHSC.
MCC presentation
The characteristics for all patients, stratified by MCC presentation, are displayed in Table 1. Patients in both groups had similar age, co-morbidity index, and gender. There was no significant difference in the proportion of patients requiring major liver resection. While patients presented at MCC were more likely to have a rectal cancer, this was not statistically significant (59% vs. 35%, P=0.082). Patients who were presented at MCC were significantly more likely (P≤0.0001) to undergo simultaneous resection of the primary colorectal tumor and liver metastases. Of the remaining patients who did not undergo simultaneous resection, 2 (25%) of those presented at MCC underwent colorectal surgery first, while 6 had a liver-first approach. In contrast, of the patients who were not presented at MCC and did not have simultaneous resection, 25 (93%) had colorectal surgery first, while 2 underwent a liver-first approach.
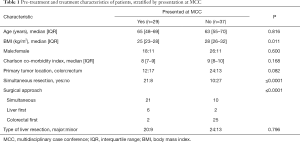
Full table
Surgical approach
Thirty-one patients (47%) underwent simultaneous resection. The characteristics of all patients are displayed in Table 2. The two groups had similar age, BMI, and gender; however, patients who underwent simultaneous resection had a significantly lower co-morbidity index (P=0.036). There were no significant differences in the proportion of patients with rectal cancer, or the proportion who required a major liver resection.

Full table
Perioperative chemotherapy and radiation
Treatment details, including the number of patients receiving neoadjuvant and adjuvant therapy, are displayed in Table 3. Few patients (20%) who underwent a colorectal-first approach without presentation at MCC received neoadjuvant chemotherapy. In contrast, most patients presented at MCC received neoadjuvant chemotherapy (25/29, 86%) and adjuvant chemotherapy (27/29, 93%). Overall, there was a trend towards increased use of neoadjuvant and adjuvant chemotherapy after MCC presentation; however, no statistically significant differences were found.
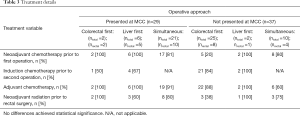
Full table
Discussion
Our analysis of patients with synchronous colorectal cancer and liver metastases revealed that patients presented at a MCC underwent a greater diversity of treatment approaches, suggesting more individualized care. More specifically, these patients were significantly more likely to undergo a simultaneous or liver-first surgical approach. Of note, there was also a trend towards a greater proportion of patients receiving chemotherapy and radiation following presentation at MCC.
Presentation of cancer cases at MCCs has become a common component of cancer care at tertiary centers. Cancer Care Ontario, the provincial body which sets mandates and guidelines for oncology care in Ontario, has recommended that all cancer patients in Ontario should have the opportunity of MCC review, irrespective of their geographical location in the province (20). Multidisciplinary team management, in the context of multidisciplinary clinics, has been shown to improve the process of care and long-term outcomes for patients with a variety of tumor types (21-25). Existing evidence supports centralisation of care for patients with rectal cancer, demonstrating improved oncological outcomes due to multidisciplinary management (26-28). Furthermore, the Consortium for Optimizing Surgical Treatment of Rectal Cancer (OSTRiCh) group has strongly advocated for MCC discussion of patients with rectal cancer in the United States in an attempt to achieve improvements and standardization in quality of rectal cancer care (29). This group’s objectives have been supported by the Commission on Cancer® and while they do not specifically focus on MCC for the management of stage IV disease, this endorsement highlights the essential role of MCC in colorectal cancer care.
While few large studies have focused on the specific role and impact of MCCs, several smaller studies have demonstrated significant changes in decision-making and treatment planning as a result of MCC contribution. Changes in the management of head and neck (2), gastroesophageal (3), colon (4), breast (5), lung (6), gynecologic (7), and urologic (8) malignancies have been found following multidisciplinary review. Similarly, a study of 641 patients with colorectal cancer found a modest proportion of patients whose treatment plans were altered following MCC review (9); however, the impact of MCC discussion on long-term oncological outcome remains unknown.
Despite the weekly opportunity for presentation at MCC, fewer than half of the patients in our cohort were presented at MCC prior to surgery and only 14% of cases were presented from regional community hospitals. Consequently, almost half of patients who underwent elective surgery for their primary tumor without a preceding MCC discussion were operated on in a community setting prior to referral for management of liver metastases, and only one fifth of those patients received neoadjuvant chemotherapy prior to the colorectal surgery. In general, patients who were not presented at MCC followed a typical colorectal-first approach to treatment and many of these unpresented patients may have had their care plan significantly altered had they been presented at a MCC. While superiority of the simultaneous or liver-first approach remains the subject of active debate, the variability in care plans recommended by the MCC suggests greater individualization of care which is hypothesized to improve treatment outcomes. Currently, there is limited understanding as to why such few patients with stage IV disease from community hospitals, and less than half in the overall series, were discussed at MCC. This finding warrants further investigation since it exists in the context of videoconference availability at all surrounding community sites. Facilitators and barriers to MCC presentation must be thoroughly examined as they may be crucial areas for quality improvement. Resources may be better allocated to ensure that teleconferencing from various community hospitals can be coordinated at a time convenient to all specialists involved.
Some limitations arise due to the design of this study. Due to its retrospective nature, it is unclear whether MCC presentation was responsible for the differences in management, or whether confounding variables are the underlying impetus for both MCC participation and varying treatment approaches. Of note, no difference was found in location of the primary tumor or extent of liver resection required for patients presented at MCC compared to those who were not discussed, suggesting that the tumor burden of the groups was fairly equivalent. Additionally, the modest sample size of the cohort precludes an in-depth analysis of the long-term oncological outcomes following simultaneous versus two-staged resection of colorectal cancer and associated liver metastases; however this question has been addressed in large meta-analyses, with no significant differences in survival identified (30-32). Furthermore, while simultaneous colorectal resection and major liver resection should be approached with caution, it has been performed safely in experienced centers (33-35) and may be considered in order to minimize the number of surgeries and overall length of treatment. While we did not have access to complete data on total treatment time for patients who had the colon portion of their staged procedure performed outside of our local area, we suspect that total time spent receiving treatment may be shorter for synchronous resections. Moreover, the rational for a simultaneous approach includes eliminating the possibility of short-interval disease progression during the inter-stage period of the colorectal-first approach, simplifying adjuvant chemotherapy by eliminating the inter-stage period during which the value of additional cycles is unknown, reducing length of hospital stay, minimizing cost of care, and reducing perioperative morbidity in a proportion of patients. Since no experimental studies have been performed comparing the surgical approaches, these hypotheses remain unproven. Nonetheless, they represent a myriad of significant potential benefits for those who are candidates for synchronous resections, and should be further studied.
Conclusions
This study examined whether the management of patients with synchronous colorectal primary tumors and liver metastases was influenced by MCC discussion. The results suggest that patients who were presented at a MCC were more likely to undergo a variable treatment course, while unpresented cases followed a typical colorectal-first pattern. In light of these findings, we suggest that all patients with known or suspected resectable CRLM be presented at a regional MCC in order to facilitate individualization of treatment plans. Until further evidence identifies a definitively superior treatment approach, patients should have equal access to consideration of all potential options including induction and adjuvant chemotherapy, as well as a non-traditional sequencing of operations. Based on our data, we anticipate that presentation of these patients at MCC may alter the surgical approach in a significant proportion of cases, and potentially result in a larger percentage of patients receiving neoadjuvant and adjuvant therapy. Further studies are needed to assess whether MCC discussion improves treatment outcomes for this unique patient population.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Western University Office of Research Ethics and the Lawson Health Research Institute (REB # 106917).
References
- Adam R, de Gramont A, Figueras J, et al. Managing synchronous liver metastases from colorectal cancer: A multidisciplinary international consensus. Cancer Treat Rev 2015;41:729-41. [Crossref] [PubMed]
- Wheless SA, McKinney KA, Zanation AM. A prospective study of the clinical impact of a multidisciplinary head and neck tumor board. Otolaryngol Head Neck Surg 2010;143:650-4. [Crossref] [PubMed]
- van Hagen P, Spaander MCW, van der Gaast A, et al. Impact of a multidisciplinary tumour board meeting for upper-GI malignancies on clinical decision making: a prospective cohort study. Int J Clin Oncol 2013;18:214-9. [Crossref] [PubMed]
- Kehl KL, Landrum MB, Kahn KL, et al. Tumor Board Participation Among Physicians Caring for Patients With Lung or Colorectal Cancer. J Oncol Pract 2015;11:e267-78. [Crossref] [PubMed]
- Newman EA, Guest AB, Helvie MA, et al. Changes in surgical management resulting from case review at a breast cancer multidisciplinary tumor board. Cancer 2006;107:2346-51. [Crossref] [PubMed]
- Ung KA, Campbell BA, Duplan D, et al. Impact of the lung oncology multidisciplinary team meetings on the management of patients with cancer. Asia Pac J Clin Oncol 2016;12:e298-304. [Crossref] [PubMed]
- Greer HO, Frederick PJ, Falls NM, et al. Impact of a weekly multidisciplinary tumor board conference on the management of women with gynecologic malignancies. Int J Gynecol Cancer Off J Int Gynecol Cancer Soc 2010;20:1321-5.
- Kurpad R, Kim W, Rathmell WK, et al. A multidisciplinary approach to the management of urologic malignancies: does it influence diagnostic and treatment decisions? Urol Oncol 2011;29:378-82. [Crossref] [PubMed]
- Fernando C, Frizelle F, Wakeman C, et al. Colorectal multidisciplinary meeting audit to determine patient benefit. ANZ J Surg 2015. [Epub ahead of print]. [Crossref] [PubMed]
- Minagawa M. Selection Criteria for Simultaneous Resection in Patients With Synchronous Liver Metastasis. Arch Surg 2006;141:1006. [Crossref] [PubMed]
- Cummings LC, Payes JD, Cooper GS. Survival after hepatic resection in metastatic colorectal cancer: A population-based study. Cancer 2007;109:718-26. [Crossref] [PubMed]
- Manfredi S, Lepage C, Hatem C, et al. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg 2006;244:254-9. [Crossref] [PubMed]
- Ejaz A, Semenov E, Spolverato G, et al. Synchronous primary colorectal and liver metastasis: impact of operative approach on clinical outcomes and hospital charges. HPB 2014;16:1117-26. [Crossref] [PubMed]
- Worni M, Mantyh CR, Akushevich I, et al. Is There a Role for Simultaneous Hepatic and Colorectal Resections? A Contemporary View from NSQIP. J Gastrointest Surg 2012;16:2074-85. [Crossref] [PubMed]
- Adam R. Colorectal cancer with synchronous liver metastases. Br J Surg 2007;94:129-31. [Crossref] [PubMed]
- Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet 2008;371:1007-16. [Crossref] [PubMed]
- Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731-40. [Crossref] [PubMed]
- Henson DE, Frelick RW, Ford LG, et al. Results of a national survey of characteristics of hospital tumor conferences. Surg Gynecol Obstet 1990;170:1-6. [PubMed]
- Reddy SK, Barbas AS, Turley RS, et al. A standard definition of major hepatectomy: resection of four or more liver segments. HPB (Oxford) 2011;13:494-502. [Crossref] [PubMed]
- Wright F, De Vito C, Langer B, et al. Multidisciplinary Cancer Conference Standards. Toronto (ON): Cancer Care Ontario; 2006 Jun 1 Program in Evidence-based Care Evidence-based Series MCC Standards Special Report.
- Gabel M, Hilton NE, Nathanson SD. Multidisciplinary breast cancer clinics: Do they work? Cancer 1997;79:2380-4. [Crossref] [PubMed]
- Chang JH, Vines E, Bertsch H, et al. The impact of a multidisciplinary breast cancer center on recommendations for patient management: The University of Pennsylvania experience. Cancer 2001;91:1231-7. [Crossref] [PubMed]
- Stephens MR, Lewis WG, Brewster AE, et al. Multidisciplinary team management is associated with improved outcomes after surgery for esophageal cancer. Dis Esophagus 2006;19:164-71. [Crossref] [PubMed]
- Birchall M, Bailey D, King P. Effect of process standards on survival of patients with head and neck cancer in the south and west of England. Br J Cancer 2004;91:1477-81. [PubMed]
- Pawlik TM, Laheru D, Hruban RH, et al. Evaluating the Impact of a Single-Day Multidisciplinary Clinic on the Management of Pancreatic Cancer. Ann Surg Oncol 2008;15:2081-8. [Crossref] [PubMed]
- Bülow S, Harling H, Iversen LH, et al. Improved survival after rectal cancer in Denmark. Colorectal Dis 2010;12:e37-42. [PubMed]
- Wibe A, Møller B, Norstein J, et al. A National Strategic Change in Treatment Policy for Rectal Cancer—Implementation of Total Mesorectal Excision as Routine Treatment in Norway. A National Audit Dis Colon Rectum 2002;45:857-66. [Crossref] [PubMed]
- Khani MH, Smedh K. Centralization of rectal cancer surgery improves long-term survival. Colorectal Dis 2010;12:874-9. [Crossref] [PubMed]
- Dietz DW, Consortium for Optimizing Surgical Treatment of Rectal Cancer. (OSTRiCh). Multidisciplinary management of rectal cancer: the OSTRICH. J Gastrointest Surg 2013;17:1863-8. [Crossref] [PubMed]
- Slesser AAP, Simillis C, Goldin R, et al. A meta-analysis comparing simultaneous versus delayed resections in patients with synchronous colorectal liver metastases. Surg Oncol 2013;22:36-47. [Crossref] [PubMed]
- Yin Z, Liu C, Chen Y, et al. Timing of hepatectomy in resectable synchronous colorectal liver metastases (SCRLM): Simultaneous or delayed? Hepatology 2013;57:2346-57. [Crossref] [PubMed]
- Feng Q, Wei Y, Zhu D, et al. Timing of Hepatectomy for Resectable Synchronous Colorectal Liver Metastases: For Whom Simultaneous Resection Is More Suitable - A Meta-Analysis. PLoS One 2014;9:e104348. [Crossref] [PubMed]
- Muangkaew P, Cho JY, Han HS, et al. Outcomes of Simultaneous Major Liver Resection and Colorectal Surgery for Colorectal Liver Metastases. J Gastrointest Surg 2016;20:554-63. [Crossref] [PubMed]
- Karoui M, Vigano L, Goyer P, et al. Combined first-stage hepatectomy and colorectal resection in a two-stage hepatectomy strategy for bilobar synchronous liver metastases. Br J Surg 2010;97:1354-62. [Crossref] [PubMed]
- Capussotti L, Ferrero A, Viganò L, et al. Major liver resections synchronous with colorectal surgery. Ann Surg Oncol 2007;14:195-201. [Crossref] [PubMed]


