Regional hepatic therapies: an important component in the management of colorectal cancer liver metastases
Introduction
Surgical resection is the only potentially curative treatment option for patients with colorectal cancer liver metastases (CRLM). Unfortunately up to 80% of these patients will present with unresectable metastatic liver disease (1,2). Five year survival for resectable patients is reported to range from 40-58% (3,4) while for unresectable patients the median overall survival is reported to be 15 to 22 months (5). Over the last 15 years, the development of new systemic chemotherapy, targeted biologic agents, as well as regional hepatic therapies (RHT) including ablative technologies and trans-arterial treatments have expanded the management options for patients with CRLM. The use of these RHT has created a paradigm shift in the treatment of CRLM, such that the historical perspective of outcome limited to cure or failure has been replaced by the more dynamic concept of converting cancer to a manageable chronic disease.
A multimodal and multidisciplinary approach is necessary to offer optimal individualized treatment. Defining the appropriate sequence and combination of treatments is challenging and requires both expertise and experience. This article reviews the currently available RHT options for unresectable CRLM and offers management strategies for this group of challenging patients.
Determining CRLM resectability
The definition of resectable CRLM has evolved significantly over the last two decades. The classic resection criteria were based on the number and size of liver lesions. Currently, resectable CRLM are more broadly considered to be any hepatic tumors that can be removed with negative margins while leaving a sufficient volume of functional parenchyma. Patients whom are deemed to be ineligible for CRLM resection at presentation can be considered to be unresectable or potentially resectable. Clearly unresectable patients are those with diffuse liver involvement or multiple extrahepatic sites. Such patients require systemic chemotherapy and are unlikely to be down-staged to resectable status. The potentially resectable candidates are those that have a reasonable expectation for a treatment response sufficient to enable CRLM resection with or without RHT following systemic treatment. The initial French experience reported CRLM down- staging rates of 13-16% (6). These rates have increased more recently with novel systemic therapy regimens. The Italian study by Masi et al. showed that approximately 20% of unresectable patients could be down-staged to resectable status (7) after systemic chemotherapy. Nuzzo et al., using irinotecan-based regimens, found a 35% rate of conversion (8) similar to the 36% found by Falcone et al. using FOLFOXIRI (9). The optimal chemotherapy combination for the purpose of CRLM down-staging has not been defined and the response rates vary depending on patient characteristics. Novel therapeutics agents and their combination with targeted therapy promise to improve response rates and conversion to resectability in CRLM.
Neoadjuvant chemotherapy for resectable disease
Given the significant improvement in overall survival with the use of modern systemic agents, interest in defining the role for neoadjuvant chemotherapy in resectable CRLM has emerged. However, the only randomized trial to date is the EORTC Intergroup trial 40983, which demonstrated an increase in recurrence free survival but not overall survival (10). In addition, the rate of complications following surgery was significantly increased in patients that received perioperative chemotherapy. The advantages and disadvantages of using the various neoadjuvant chemotherapy and targeted molecular therapy options for the treatment of CLRM is beyond the scope of this review. However, it is important when considering neoadjuvant chemotherapy for resectable disease to proceed in a multidisciplinary approach with the active involvement of the surgical team. It has been well documented that 4-5% of CRLM will disappear on imaging subsequent to systemic therapies, thus making post-treatment surgical resection planning difficult (11). Moreover, it is well established that a complete clinical response on imaging does not correlate with pathological response, with up to 80% of patients having positive microscopic disease (12). This highlights the importance of early referral to a liver surgeon within the context of a multidisciplinary approach.
Staged hepatectomy
Staged hepatectomy with or without portal vein embolization (PVE) is a therapeutic approach that can be considered for patients with bilateral CRLM. The use of PVE for staged hepatectomy has been demonstrated to have acceptable morbidity and mortality (13). After the initial resection, PVE is performed if necessary. The liver is allowed to hypertrophy for 3-4 weeks and then a second stage resection can be performed. The type of resection done for the first and second stages depends on the distribution and location of the liver metastases and the liver remnant volume. Although this technique has been reported only in highly specialized centers, it is a feasible option for otherwise unresectable disease.
Unresectable CRLM
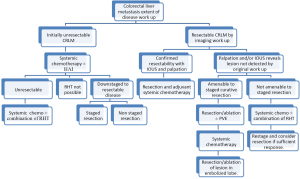
Unresectable patients can be further divided in two groups: (I) patients that after systemic and/or biological agents alone or in combination can be down-staged to resectable; and (II) the group that after systemic and/or biological agents alone or in combination cannot be down-staged to resectable status. After re-staging, patients in the first group should undergo resection to clear all hepatic disease. For the second group, RHT have emerged as part of the armamentarium to reduce or stabilize disease burden in the liver as stand-alone therapy or in combination with other modalities (Figure 1). RHT can be grouped into three broad categories: ablative, arterial and non-arterial modalities. Each of these categories can be further grouped by type of technology use to obtain cancer cells demise (Figure 2).
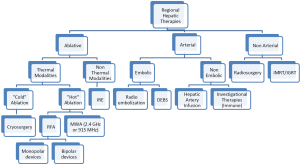
Ablative modalities
As mentioned, ablative options for unresectable CRLM can be divided into thermal and non-thermal modalities. Thermal options can be further divided in “cold” and “hot” ablation modalities. Cold ablation therapies include cryo-ablation and hot ablation modalities include radiofrequency ablation (monopolar and bipolar devices), microwave ablation (2.45 GHz and 915 MHz). Another type of ablation used in the past is chemical ablation, but its use has been abandoned with the emergence of new, more effective, and easier to use modalities. The principal non-thermal option is irreversible electroporation.
Thermal “hot” ablation
Radiofrequency ablation (RFA)
Radiofrequency ablation energy can be delivered by either monopolar or bipolar devices. Monopolar is the most frequently used, and consist of an electrode that streams energy outwardly in all directions. The radius of tissue necrosis varies and depends in the type and configuration of the electrode used. The other RFA modality is bipolar, which consist of two parallel electrodes facing one another. In bipolar systems, the energy travels between the electrodes and not around them, concentrating energy delivery into the area between the electrodes.
Monopolar RFA
RFA is presently the most common “hot” ablative therapy. RFA induces tumor necrosis by achieving local hyperthermia with temperatures exceeding 58 °C. The energy for RFA is based on an alternating current of radio frequency waves (500 kHz) that is delivered via a probe into the tissue being treated. The resulting ionic agitation generates frictional heat which extends to adjacent tissue by conduction leading to coagulative necrosis (14). RFA probes may be deployed via open, percutaneous, or laparoscopic approaches. The optimal approach depends on tumor location and the operator preference. Several studies (15-17) have shown lower local recurrence rate with the open approach. Better exposure of the liver, the ability to visually inspect and palpate liver surface lesions, combined with the use of intra-operative ultrasound may explain the superior results of the open approach (18).
In the last decade, radiofrequency ablation has superseded other ablative therapies, due to its low morbidity, low mortality, and technical feasibility (19). However, is very difficult to analyze the available RFA data, in terms of local recurrence, overall survival and progression free survival. Most of the published studies are observational or clinical trials with no randomization, resulting in potential biases that make comparison of groups difficult. Heterogeneity of the treatments approaches further confounds the situation, making it difficult to draw conclusions (19). Despite these difficulties, several studies exist that support the following:
Local recurrence and intrahepatic progression free survival
Progression of intra-hepatic disease and local recurrence has been related to survival of patient with unresectable disease. RFA as standalone therapy or in combination with other modalities is a useful tool to obtain hepatic disease control. Location of the lesion is also associated with an increase in local recurrence after RFA. Lesions close to major hepatic vessels have a higher local recurrence rate due to the decrease in temperature in the ablation area because of blood flow. The local recurrence rates for current RFA technology have been reported as 9-21% (20,21) and this have been associated with tumor size and location of the lesion. With the current RFA probes lesions up to 5 cm are suitable for RFA (22) with higher rates of local recurrence with lesions >5 cm (23).
Overall survival
The 2 year survival rates with classic fluorouracil based regimens have been reported between 22-27% (19). When RFA is used with chemotherapy, the 2, 3 and 5 years survival rates reported are 60%, 34% and 22% respectively, with more recent studies presenting 5 years survival rates between 25-31% (24,25). This data needs to be evaluated carefully because, as mentioned before, most of the studies lack randomization and have high risk of selection bias. While RFA may contribute to improved outcomes in certain situations, increased survival when RFA is added to systemic therapy may be in part due to selection of patients with less extensive disease, amenable to ablation. Randomized clinical trials powered to measure overall survival are required.
Bipolar RFA
Radiofrequency ablation is the most widely accepted and available ablative modality. However, is limited by inconsistent ablation zones, susceptibility to convective heat loss from adjacent high-velocity blood flow or heat sinks (26,27). In an effort to resolve these problems, other RFA ablative configurations have emerged. Bipolar RFA is one such modality, which employs a dual parallel electrode array; the energy wave travels uni-directionally between and not around electrodes. This ‘line-of-sight’ delivery streams energy between two fixed points and concentrates energy delivery to the area between the probes (27).
The use of two electrodes with very high current density decreases the time required to achieve target temperature in treated tissue in comparison to monopolar devices (27). Convective heat loss seems to be negligible with bipolar RFA. One of the limitations of this technique is that the ablation area is defined by the orientation between the electrodes. If the electrodes are not parallel to each other the ablation area could take unpredictable shapes resulting in unintended thermal injury to noninvolved tissue (26,28). The operator needs to understand the characteristics of the device to obtain the desired ablation shape. The key aspect when planning the ablation area is to define the perimeter of the ablation target lesion.
In a report published by Baldwin et al. (28) 22 patients were treated with bipolar RFA, and after a median follow up of 24 months only one patient showed local recurrence. The time of ablation was 4-7 min with increased ablation time associated with lesion size. Although this is a small series with CRLM and hepatocellular carcinoma (HCC), it demonstrated that bipolar RFA can be used with acceptable results and the laparoscopic approach is not technically challenging. Bipolar RFA is a technology in its infancy and further studies with longer follow-up will be required to establish the long-term oncologic outcomes for this technique. However, the rapid emergence of microwave technology may lead to diminished utilization of RFA, as described below.
RFA in combination with systemic chemotherapy
The use of RFA with systemic chemotherapy has been recently studied in the EORTC 40004 trial. This is currently the only clinically randomized control trial, comparing RFA + systemic chemotherapy to systemic chemotherapy alone for unresectable CRLM (29). This originally planned phase III trial was downgraded to phase II because of slow accrual. The data presented is consistent with previous non-randomized studies, where the combination group (RFA + systemic chemotherapy) had a significantly higher progression free survival at 3 years; 27.6% vs. 10% in the systemic chemotherapy only group. Unfortunately, the study was not powered for overall survival. However, a trend towards increased overall survival was seen in the combination group. Appropriately powered studies will be necessary to determine if there is a difference in overall survival (29).
RFA plus resection
The combination of resection and RFA may enable complete intrahepatic tumor clearance under circumstances where resection alone would not leave sufficient remnant liver. Several non-randomized studies (24,30) have shown the use of RFA as a complement to resection may enable complete tumor clearance. No statistically significant difference in overall survival has been observed between RFA + resection and RFA alone (31), suggesting that RFA is a reasonable adjunct to liver resection in selected cases. Ablation therapies appear to be a promising adjunct to resection in patients that otherwise would be rendered free of disease by resection alone.
Microwave ablation
Microwave ablation (MWA) is also dependent on thermal energy. MWA utilizes the region of electromagnetic spectrum between 915 MHz and 2.4 GHz. When the microwaves interact with water molecules, frictional heat is generated, resulting in coagulative necrosis (32). Gravante et al. (33) studied pathology specimens after the use of MWA and found no viable tumor cells in 93% of lesions ≤6 cm in diameter.
The use of MWA has been more prevalent in Asia than in the USA, were RFA has been the more commonly used thermal ablation modality. Although most of the data comes from interventions in unresectable HCC patients (34-36), MWA has been demonstrated to be a safe technique with similar morbidity to RFA (35,36). Morita et al. (37) reported an experience with 52 patients with CRLM using MWA alone and in combination with resection. For these groups the cumulative 5-year survival rates were similar, at 20% and 24%, respectively. These data are comparable with the long-term survival found after RFA alone or with resection (19). As with RFA, the risk of local recurrence following MWA is higher for lesions larger than 5cm (36,38). A randomized study is necessary to find if any difference in local recurrence and overall survival exist between MWA and RFA.
Irreversible electroporation (IRE)
The known limitations of RFA and MWA such as biliary tract damage, heat-sink effect, and thermal damage to adjacent organs have led to the pursuit of alternative ablation technologies. Irreversible electroporation (IRE) is a novel technology that has been proposed to improve the ablation efficacy around major portal or hepatic vessels. In contrast to RFA and MWA, IRE employs electrical pulses that permeabilize cellular membrane and consequently lead to cell death (39). These electrical impulses create nanopores in both normal and malignant cells. The collagen scaffolding of structures such as vessels and biliary structures do not form nanopores and therefore are not affected by IRE. One of the hypotheses for this “sparing effect” holds that gap junctions in vessels walls allow the electrical impulse to transfer from one cell to the other without affect (40,41). One of the advantages of this modality is the ability to cause cell death in the hepatic parenchyma around major hepatic vessels, avoiding the “sink effect” seen with the use of RFA or MWA.
IRE has shown to be safe in porcine liver models and recently the first series in humans have been published (42). A recent retrospective study from the Memorial Sloan-Kettering Cancer Center (MSKCC) demonstrated tumor response rates of 98%, which was higher than the 50% rate reported in another studies (43). The MSKCC group utilized an open approach, which may account for the higher response rates. The percutaneous approach has the potential limitation of positioning accuracy of the IRE electrodes, while the open approach facilitates a more accurate positioning of the electrodes aided by palpation. Recurrence rates of 5.7% were seen with 6 months median follow up. Although the study has various limitations including selection bias, short follow-up and tumor type heterogeneity, it demonstrates that IRE can be done safely and with promising results. Larger series with long-term follow up will be required to validate IRE as an effective regional liver therapy modality for liver tumors.
Arterial modalities
Arterial modalities can be divided in embolic and non-embolic. Embolic therapies include selective internal radiation therapy (SIRT), drug eluding beads (DEBS) and trans-arterial chemo-embolization. Non-embolic treatments include hepatic artery infusion of chemotherapy and regional adoptive cellular immunotherapy, which is currently under study at our institution.
Selective internal radiation therapy (SIRT)
Yttrium 90 (Y90) is the most common agent used for SIRT, a new option for patients with unresectable CRLM. This modality can be used as a single therapy for chemotherapy refractory patients or in combination with systemic chemotherapy. Y90 is a pure beta-emitting radioisotope, produced by the bombardment of Y89 with neutrons. Y90 has a high average energy (0.936 MeV), limited tissue penetration (mean 2.5 mm, max 11 mm), and short half-life (64 n), making it an ideal trans-arterial liver-directed agent. After incorporation into glass or resin microspheres, Y90 is selectively injected into the hepatic artery or its branches (44). There are two commercially available forms of Y90 microsphere: SIR-Spheres (Sirtex Medical, Sydney, Australia) and TheraSphere (MDS Nordion, Ontario, Canada). SIR-Spheres are resin-based microsphere, have a diameter of 20-60 µm. SIR-Spheres are used mainly in the treatment of CRLM and received pre-market approval by the FDA in 2002. TheraSphere are made of glass and have a diameter of 20-30 µm, used more frequently in HCC treatment, for which it has a humanitarian device exemption.
Important in the use of Y90 microspheres is the pre-therapy planning. All patients considered Y90 internal radiation must undergo hepatic angiography and a technetium-99 macroaggregated albumin (Tch-99 MAA) nuclear medicine scan. The goal of this assessment is to delineate the hepatic arterial vasculature and quantify the degree of extrahepatic perfusion and hepatopulmonary shunting. Infusion of radioactive microspheres into the gastrointestinal or pulmonary circulation can have devastating consequences. Thus Tch-99 MAA, which has a similar diameter as the microspheres, is used as a surrogate to estimate the distribution of the microspheres in the hepatic circulation prior to therapy. The degree of hepatopulmonary shunting and reflux into the gastrointestinal circulation can be determined. A hepatopulmonary shunt greater than 18% predisposes patients to development of radiation pneumonitis and represents a contraindication to Y90 therapy, unless the shunts can be occluded by embolization. Gastrointestinal arterial reflux that cannot be eliminated by ligation or embolization also precludes patients from undergoing treatment. Severe liver dysfunction or portal vein thrombosis are also contraindications to therapy, although patients with the latter have undergone glass microsphere treatment successfully (45,46).
The treatment response after SIRT can be measured by fluctuations in CEA levels and by imaging. The earliest published data for SIRT in unresectable CRLM examined the combination of SIRT with hepatic artery chemotherapy (HAC) (47). This data showed significantly longer median survival rates in patients receiving SIRT, with 6, 12 and 18 months estimated survival rates of 70%, 46% and 46% respectively. These survival rates were limited by the development of extrahepatic disease. There was no treatment associated mortalities and SIRT was well tolerated. Later studies also from New Zealand and Australia have shown significant difference in tumor response and median survival time in patients who receive SIRT in addition to HAC (48,49) with mean CEA level drop of 50-70% of pre-treatment levels, and greater than 50% reduction in tumor volume. Data gathered form a phase III randomized clinical trial by Gray et al. (50) in 2001 showed significantly better tumor response in the SIRT + HAC group than in the HAC alone group (72% vs. 47% respectively). Likewise, time to disease progression was significantly longer (15.9 vs. 9.7 months) in the SIRT + HAC group. Van Hazel et al. (51) published a RCT in 2004 evaluating SIRT alone or SIRT in combination with systemic 5-FU and leucovorin. In this small RCT the combination group had significantly higher response rates and longer time to disease progression than the chemotherapy alone group (18.6 vs. 3.6 months respectively).
Y90 seems to be safe and effective therapy for unresectable CRLM. However, the optimal dose and timing of Y90 therapy remain to be established. The use of doses greater than 225 Gy results in superior response rates and cumulative doses greater than 300 Gy led to a significantly increased survival (52,53). The precise correlation between degree of hepatic dysfunction and tolerance of radiation needs to be characterized and further randomized trials are needed to accurately define the safest and most effective dose and to determine timing between therapies. The principal determinant of survival following SIRT in CRLM is the development of extrahepatic disease. As such, combining SIRT with systemic therapy may prove to be the most rationale approach and pre-SIRT PET may be used to refine patient selection for this modality (49).
Y90 is a novel addition to the RHT for unresectable liver tumors. Sufficient data exist to support it use in unresectable CRLM, with increase in median survival, time to progression of hepatic disease and tumor response rates. The tumor response induced by Y90 radioembolization is a valuable tool for attempted conversion of unresectable to resectable disease. Further studies are needed to assess the optimal Y90dose, indications, and its place alongside the other RHT.
Hepatic arterial chemotherapy
Hepatic arterial infusion chemotherapy is another modality within the RHT used in combination with systemic chemotherapy, to induce greater tumor response and ultimately longer median survival. The rationale behind hepatic artery infusion chemotherapy is based on the principle that CRLM get their blood supply almost exclusively from the hepatic artery, while the normal liver parenchyma receive the majority of its blood supply from the portal vein (54). Thus prolonged drug exposure and higher concentrations in CRLM can be achieved with direct hepatic artery infusion of the chemotherapeutic agents, with limited systemic toxicity.
The use of HAC was first studied alone, and response rates were reported to be between 22% and 62% (55,56). Later the use of HAC in combination with systemic chemotherapy gained more popularity because the tumor response rates were better and better control of extrahepatic disease was possible (57,58). The tumor response seen with the combination of HAC + systemic chemotherapeutics ranged from 35% to 92%. Most of the studies used 5 FU/Leucovorin as systemic therapy, however higher response rates were seen with Oxaliplatin/Irinotecan regimens (54). HAC + systemic chemotherapy have shown to be a valuable tool to achieved resectability, with rates of resectability in the 50% range when used as first line therapy and 20% after failure with systemic chemotherapy (59).
Complications from HAC have discouraged its use, these are related with the drug itself or technical. Allen et al. published his experience at MSKCC with and overall pump complication rate of 22%. Complications such as arterial thrombosis (6%), extrahepatic perfusion (3%), incomplete hepatic perfusion (2%) and hemorrhage (2%) were reported. However, these technical complications improved with increased experience, with significantly lower rates in the second half of the study (60). From the drug related complications the most common and serious is hepatobiliary toxicity. Usually one of the first signs will be elevation of transaminases levels, while elevation of bilirubin and alkaline phosphatase show signs of more significant hepatic damage. Dose-adjusting algorithms have been developed based on changes in the liver function tests to better guide the dosage and avoid toxicity and dexamethasone have also been added to reduce the incidence on biliary toxicity (61).
In the last decade, several studies have provided data about the use of HAC therapy in patients with unresectable CRL, most of them using floxuridine. The combination with modern systemic chemotherapeutics (oxaliplatin/irinotecan based regimens) has further increased the tumor response. Impressive tumor response rates of 92% have been published when HAI is combined with modern systemic chemotherapy, with resection rates of 47-53% when used as first line therapy and median survival of 51 months for chemotherapy-naïve patients and 35 months for previously treated (62). Major complications are associated with HAI; hepatotoxicity and technical problems with the delivery systems limit their use to only a few centers in the world with enough experience to provide this highly specialized treatment.
Drug eluting beads (DEBS)
Drug eluting beads or DEBS have emerged as a tool to deliver chemotherapeutic agents to a specific area, decreasing the release into non-target regions (63). This facilitates higher doses to tumor cells, limiting the dose to the normal liver parenchyma and extrahepatic sites, decreasing toxicity. The agent is embedded in beads enough to minimize diffusion by embolizing the terminal capillaries. Modern angiographic techniques can deliver these beads directly to the tumor with low complication risk.
Recent reports have shown that DEBS therapy is well tolerated by patients (64,65). Major risks include liver failure and gastric irritation caused by seepage into the gastrointestinal tract; initial studies have demonstrated this technique to be safe in the treatment of CRLM (66). Post embolic syndrome, consisting of nausea, vomiting, dehydration and pain, have been reported in patients receiving multiple treatments with cumulative doses higher than 300 mg (67).
The chemotherapeutic agents used for DEBS have changed with the initial reports describing use of mitomycin C in combination with methylcellulose microcapsules. The more recent studies report Irinotecan (DEBIRI) with doses ranging from 50 to 200 mg per treatment. Data from studies using DEBIRI are difficult to analyze for several reasons: not all the patients received the same chemotherapeutic, the early trials used mitomycin C and the later irinotecan, different number of treatments were used and most of the patients had already failed different systemic treatments or other loco-regional therapies had been used. Martin et al. (67) showed tumor response rates with DEBIRI of 73% at 3 months, 56% at 6 months and 40% at 12 months using the RECIST criteria, with a median overall survival of 343 days and median free-survival was 197 days. DEBS is a therapy that is in its infancy and further studies are necessary to better understand the possible benefits and its role in the treatment of CRLM.
Non-arterial modalities
Radiation therapy for colorectal liver metastasis has gained importance in the treatment algorithm in the last few years. The better understanding of liver tolerance to radiation and new techniques to deliver the radiation have played an important role in decreasing toxicity and improved accuracy of radiation therapy.
Pioneer studies combining intensity-modulated radiation therapy (IMRT) with image-guided radiation therapy (IGRT) by megavoltage computed tomography scanning, have shown to be safe and efficient treating CRLM. Grade 2 and 3 toxicity was reported only in 9% and 4% of the patients respectively (68). A phase II trial by Engels et al. (69) using the helical tomotherapy (IMRT + IGRT) moderately hypofractionated therapy (10 fractions of 5Gy) was used in 53 patients. Results showed tumor response rate of 55%, with actuarial 1-year local control of 54%, progression free-survival of 14% and overall survival of 78%. The local control rates presented are lower than other reported in the literature with 2-year local control of 67% (70) this is probably because the higher doses used, however to obtain higher local control rates doses >100 Gy needs to be administered and this is only possible with tolerable toxicity in <3 lesion, <4 cm in diameter and far from hollow viscus organs (69).
Radio surgery (Cyber or Gamma knife) technology has emerged as a delivery method capable to deliver high doses of radiation in a very accurate manner, compensating for respiratory movements and with a tracking system to avoid toxicity to adjacent tissues. Up to date we have not found any report studying radiosurgery for CRLM.
Conclusions
The management of unresectable CRLM is constantly evolving, with demonstrated advances in systemic chemotherapy regimens, novel biologic agents, multiple ablation modalities and more accurate radiation delivery systems. With the increasing number of potentially effective therapies and combination therapies the management of this group of patient has become very complex, and requires a well-coordinated multidisciplinary team to achieve optimal outcomes. Selecting the best next therapy for each patient should be individualized and modeled to the different characteristics of each patient and tumor biologic features. In the coming years, randomized clinical trials will potentially offer the information necessary to assess the various RHT options alone and in combination with systemic modalities to better define the choice and sequence for their use in the treatment of the complex CRLM patient. While surgery remains the only curative approach for patients with CRLM, the majority of patients cannot be completely resected and RHT offer an important adjunct to control intrahepatic tumor burden.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Kemeny N. Management of liver metastases from colorectal cancer. Oncology (Williston Park) 2006;20:1161-76, 1179; discussion 1179-80, 1185-6.
- Van Cutsem E, Nordlinger B, Adam R, et al. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur J Cancer 2006;42:2212-21.
- Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999;230:309-18; discussion 318-21.
- Scheele J, Stangl R, Altendorf-Hofmann A. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg 1990;77:1241-6.
- Grothey A, Sargent D, Goldberg RM, et al. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol 2004;22:1209-14.
- Adam R, Wicherts DA, de Haas RJ, et al. Patients with initially unresectable colorectal liver metastases: is there a possibility of cure? J Clin Oncol 2009;27:1829-35.
- Masi G, Loupakis F, Pollina L, et al. Long-term outcome of initially unresectable metastatic colorectal cancer patients treated with 5-fluorouracil/leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) followed by radical surgery of metastases. Ann Surg 2009;249:420-5.
- Nuzzo G, Giuliante F, Ardito F, et al. Liver resection for primarily unresectable colorectal metastases downsized by chemotherapy. J Gastrointest Surg 2007;11:318-24.
- Falcone A, Ricci S, Brunetti I, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol 2007;25:1670-6.
- Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet 2008;371:1007-16.
- Benoist S, Nordlinger B. The role of preoperative chemotherapy in patients with resectable colorectal liver metastases. Ann Surg Oncol 2009;16:2385-90.
- Benoist S, Brouquet A, Penna C, et al. Complete response of colorectal liver metastases after chemotherapy: does it mean cure? J Clin Oncol 2006;24:3939-45.
- Jaeck D, Oussoultzoglou E, Rosso E, et al. A two-stage hepatectomy procedure combined with portal vein embolization to achieve curative resection for initially unresectable multiple and bilobar colorectal liver metastases. Ann Surg 2004;240:1037-49; discussion 1049-51.
- McGahan JP, Brock JM, Tesluk H, et al. Hepatic ablation with use of radio-frequency electrocautery in the animal model. J Vasc Interv Radiol 1992;3:291-7.
- Elias D, Sideris L, Pocard M, et al. Incidence of unsuspected and treatable metastatic disease associated with operable colorectal liver metastases discovered only at laparotomy (and not treated when performing percutaneous radiofrequency ablation). Ann Surg Oncol 2005;12:298-302.
- Amersi FF, McElrath-Garza A, Ahmad A, et al. Long-term survival after radiofrequency ablation of complex unresectable liver tumors. Arch Surg 2006;141:581-7; discussion 587-8.
- Poon RT, Ng KK, Lam CM, et al. Learning curve for radiofrequency ablation of liver tumors: prospective analysis of initial 100 patients in a tertiary institution. Ann Surg 2004;239:441-9.
- Wood TF, Rose DM, Chung M, et al. Radiofrequency ablation of 231 unresectable hepatic tumors: indications, limitations, and complications. Ann Surg Oncol 2000;7:593-600.
- Hering J, Garrean S, Saied A, et al. Use of radiofrequency hepatic parenchymal transection device in hepatic hemangioma resection: early experience and lessons learned. HPB (Oxford) 2007;9:319-23.
- Gillams AR, Lees WR. Survival after percutaneous, image-guided, thermal ablation of hepatic metastases from colorectal cancer. Dis Colon Rectum 2000;43:656-61.
- de Baere T, Elias D, Dromain C, et al. Radiofrequency ablation of 100 hepatic metastases with a mean follow-up of more than 1 year. AJR Am J Roentgenol 2000;175:1619-25.
- Ahmad A, Chen SL, Kavanagh MA, et al. Radiofrequency ablation of hepatic metastases from colorectal cancer: are newer generation probes better? Am Surg 2006;72:875-9.
- Qian J. Interventional therapies of unresectable liver metastases. J Cancer Res Clin Oncol 2011;137:1763-72.
- Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg 2004;239:818-25; discussion 825-7.
- Curley SA. Outcomes after surgical treatment of colorectal cancer liver metastases. Semin Oncol 2005;32:S109-11.
- Berber E, Siperstein A. Local recurrence after laparoscopic radiofrequency ablation of liver tumors: an analysis of 1032 tumors. Ann Surg Oncol 2008;15:2757-64.
- Yi B, Somasundar P, Espat NJ. Novel laparoscopic bipolar radiofrequency energy technology for expedited hepatic tumour ablation. HPB (Oxford) 2009;11:135-9.
- Baldwin K, Haniff M, Somasundar P. Initial experience using a bipolar radiofrequency ablation device for hemostasis during thyroidectomy. Head Neck 2013;35:118-22.
- Ruers T, Punt C, Van Coevorden F, et al. Radiofrequency ablation combined with systemic treatment versus systemic treatment alone in patients with non-resectable colorectal liver metastases: a randomized EORTC Intergroup phase II study (EORTC 40004). Ann Oncol 2012;23:2619-26.
- Pawlik TM, Izzo F, Cohen DS, et al. Combined resection and radiofrequency ablation for advanced hepatic malignancies: results in 172 patients. Ann Surg Oncol 2003;10:1059-69.
- Tanabe KK, Curley SA, Dodd GD, et al. Radiofrequency ablation: the experts weigh in. Cancer 2004;100:641-50.
- Simon CJ, Dupuy DE, Mayo-Smith WW. Microwave ablation: principles and applications. Radiographics 2005;25:S69-83.
- Gravante G, Ong SL, Metcalfe MS, et al. Hepatic microwave ablation: a review of the histological changes following thermal damage. Liver Int 2008;28:911-21.
- Lu MD, Chen JW, Xie XY, et al. Hepatocellular carcinoma: US-guided percutaneous microwave coagulation therapy. Radiology 2001;221:167-72.
- Shiina S, Teratani T, Obi S, et al. Nonsurgical treatment of hepatocellular carcinoma: from percutaneous ethanol injection therapy and percutaneous microwave coagulation therapy to radiofrequency ablation. Oncology 2002;62:64-8.
- Dong B, Liang P, Yu X, et al. Percutaneous sonographically guided microwave coagulation therapy for hepatocellular carcinoma: results in 234 patients. AJR Am J Roentgenol 2003;180:1547-55.
- Morita T, Shibata T, Okuyama M, et al. Microwave coagulation therapy for liver metastases from colorectal cancer. Gan To Kagaku Ryoho 2004;31:695-9.
- Liang P, Dong B, Yu X, et al. Prognostic factors for survival in patients with hepatocellular carcinoma after percutaneous microwave ablation. Radiology 2005;235:299-307.
- Lee EW, Thai S, Kee ST. Irreversible electroporation: a novel image-guided cancer therapy. Gut Liver 2010;4:S99-S104.
- Davalos RV, Mir IL, Rubinsky B. Tissue ablation with irreversible electroporation. Ann Biomed Eng 2005;33:223-31.
- Daniels C, Rubinsky B. Electrical field and temperature model of nonthermal irreversible electroporation in heterogeneous tissues. J Biomech Eng 2009;131:071006.
- Kingham TP, Karkar AM, D’Angelica MI, et al. Ablation of perivascular hepatic malignant tumors with irreversible electroporation. J Am Coll Surg 2012;215:379-87.
- Thomson KR, Cheung W, Ellis SJ, et al. Investigation of the safety of irreversible electroporation in humans. J Vasc Interv Radiol 2011;22:611-21.
- Garrean S, Joseph Espat N. Yttrium-90 internal radiation therapy for hepatic malignancy. Surg Oncol 2005;14:179-93.
- Carr BI. Hepatic arterial 90Yttrium glass microspheres (Therasphere) for unresectable hepatocellular carcinoma: interim safety and survival data on 65 patients. Liver Transpl 2004;10:S107-10.
- Salem R, Lewandowski R, Roberts C, et al. Use of Yttrium-90 glass microspheres (TheraSphere) for the treatment of unresectable hepatocellular carcinoma in patients with portal vein thrombosis. J Vasc Interv Radiol 2004;15:335-45.
- Stubbs RS, Cannan RJ, Mitchell AW. Selective internal radiation therapy with 90yttrium microspheres for extensive colorectal liver metastases. J Gastrointest Surg 2001;5:294-302.
- Gray BN, Burton MA, Kelleher DK, et al. Selective internal radiation (SIR) therapy for treatment of liver metastases: measurement of response rate. J Surg Oncol 1989;42:192-6.
- Stubbs RS, Wickremesekera SK. Selective internal radiation therapy (SIRT): a new modality for treating patients with colorectal liver metastases. HPB (Oxford) 2004;6:133-9.
- Gray B, Van Hazel G, Hope M, et al. Randomised trial of SIR-Spheres plus chemotherapy vs. chemotherapy alone for treating patients with liver metastases from primary large bowel cancer. Ann Oncol 2001;12:1711-20.
- Van Hazel G, Blackwell A, Anderson J, et al. Randomised phase 2 trial of SIR-Spheres plus fluorouracil/leucovorin chemotherapy versus fluorouracil/leucovorin chemotherapy alone in advanced colorectal cancer. J Surg Oncol 2004;88:78-85.
- Kennedy AS, Nutting C, Coldwell D, et al. Pathologic response and microdosimetry of (90)Y microspheres in man: review of four explanted whole livers. Int J Radiat Oncol Biol Phys 2004;60:1552-63.
- Ho S, Lau WY, Leung TW, et al. Clinical evaluation of the partition model for estimating radiation doses from yttrium-90 microspheres in the treatment of hepatic cancer. Eur J Nucl Med 1997;24:293-8.
- Kanat O, Gewirtz A, Kemeny N. What is the potential role of hepatic arterial infusion chemo-therapy in the current armamentorium against colorectal cancer. J Gastrointest Oncol 2012;3:130-8.
- Kemeny NE, Gonen M. Hepatic arterial infusion after liver resection. N Engl J Med 2005;352:734-5.
- Kemeny NE, Niedzwiecki D, Hollis DR, et al. Hepatic arterial infusion versus systemic therapy for hepatic metastases from colorectal cancer: a randomized trial of efficacy, quality of life, and molecular markers (CALGB 9481). J Clin Oncol 2006;24:1395-403.
- Kerr DJ, McArdle CS, Ledermann J, et al. Intrahepatic arterial versus intravenous fluorouracil and folinic acid for colorectal cancer liver metastases: a multicentre randomised trial. Lancet 2003;361:368-73.
- Lorenz M, Müller HH. Randomized, multicenter trial of fluorouracil plus leucovorin administered either via hepatic arterial or intravenous infusion versus fluorodeoxyuridine administered via hepatic arterial infusion in patients with nonresectable liver metastases from colorectal carcinoma. J Clin Oncol 2000;18:243-54.
- Boige V, Malka D, Elias D, et al. Hepatic arterial infusion of oxaliplatin and intravenous LV5FU2 in unresectable liver metastases from colorectal cancer after systemic chemotherapy failure. Ann Surg Oncol 2008;15:219-26.
- Allen PJ, Nissan A, Picon AI, et al. Technical complications and durability of hepatic artery infusion pumps for unresectable colorectal liver metastases: an institutional experience of 544 consecutive cases. J Am Coll Surg 2005;201:57-65.
- Cohen AD, Kemeny NE. An update on hepatic arterial infusion chemotherapy for colorectal cancer. Oncologist 2003;8:553-66.
- Kemeny NE, Melendez FD, Capanu M, et al. Conversion to resectability using hepatic artery infusion plus systemic chemotherapy for the treatment of unresectable liver metastases from colorectal carcinoma. J Clin Oncol 2009;27:3465-71.
- Tang Y, Taylor RR, Gonzalez MV, et al. Evaluation of irinotecan drug-eluting beads: a new drug-device combination product for the chemoembolization of hepatic metastases. J Control Release 2006;116:e55-6.
- Fiorentini G, Aliberti C, Turrisi G, et al. Intraarterial hepatic chemoembolization of liver metastases from colorectal cancer adopting irinotecan-eluting beads: results of a phase II clinical study. In Vivo 2007;21:1085-91.
- Aliberti C, Tilli M, Benea G, et al. Trans-arterial chemoembolization (TACE) of liver metastases from colorectal cancer using irinotecan-eluting beads: preliminary results. Anticancer Res 2006;26:3793-5.
- Martin RC, Joshi J, Robbins K, et al. Transarterial Chemoembolization of Metastatic Colorectal Carcinoma with Drug-Eluting Beads, Irinotecan (DEBIRI): Multi-Institutional Registry. J Oncol 2009;2009:539795.
- Martin RC, Robbins K, Tomalty D, et al. Transarterial chemoembolisation (TACE) using irinotecan-loaded beads for the treatment of unresectable metastases to the liver in patients with colorectal cancer: an interim report. World J Surg Oncol 2009;7:80.
- Engels B, Everaert H, Gevaert T, et al. Phase II study of helical tomotherapy for oligometastatic colorectal cancer. Ann Oncol 2011;22:362-8.
- Engels B, Gevaert T, Everaert H, et al. Phase II study of helical tomotherapy in the multidisciplinary treatment of oligometastatic colorectal cancer. Radiat Oncol 2012;7:34.
- Milano MT, Katz AW, Muhs AG, et al. A prospective pilot study of curative-intent stereotactic body radiation therapy in patients with 5 or fewer oligometastatic lesions. Cancer 2008;112:650-8.


