Liraglutide’s use in treatment of non-alcoholic fatty liver: an evaluation of the non-alcoholic steatohepatitis study
Nonalcoholic fatty liver disease (NAFLD) is a leading cause of liver related morbidity and mortality and also is the second most common cause of liver transplantation in the United States (1). NAFLD is considered the liver related manifestation of metabolic syndrome and has a prevalence of 20–33%; it is associated with obesity, type 2 diabetes, dyslipidemia and metabolic syndrome. Additionally, approximately 30% of patients with NAFLD will progress to nonalcoholic steatohepatitis (NASH), which is defined as the presence of “hepatic steatosis and lobular inflammation with hepatocyte injury (ballooning) with or without fibrosis” (2). Furthermore, NASH has been found to progress to cirrhosis in 11% of affected patients over 15 years, liver failure and hepatocellular carcinoma in 7.6% of cirrhotic patients within 6–7 years (3).
American Association for the Study of Liver Diseases (AASLD) guidelines for the treatment of NASH
Current treatment recommendations for NAFLD per the AASLD (2) include lifestyle modifications including weight loss and exercise, avoidance of heavy alcohol consumption, and the use of thiazolidinediones (TZD) and vitamin E in non-diabetic patients, with some reservations due to possible toxicities. Other recommendations such as the use of omega-3 fatty acids, and statins are recommended to treat dyslipidemia, but not NASH itself. Ultimately, there are no medications recommended specifically for the treatment of NASH that show high efficacy in lessening steatosis or fibrosis.
Liraglutide study results
The study recently published by Armstrong et al. (4), “Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study” is a proof of concept study that explores the use of liraglutide in the treatment of NASH. The primary endpoints studied were resolution of steatohepatitis and prevention of worsening of fibrosis in biopsy proven NASH patients. Patients with well controlled type 2 diabetes mellitus were included in this study, but patients with excessive alcohol consumption, poor diabetic control, Child Pugh B/C cirrhotics, patients with other liver disease and patients who use certain medications (such as insulin or other molecules currently used for NAFLD treatment) were excluded. Twenty six patients were randomized to liraglutide and 26 to placebo for 48 weeks at which point the endpoints of treatment were evaluated. All patients were also given diet, exercise and weight loss recommendations as a part of standard of care. At end of treatment, all patients were re-biopsied to evaluate for degree of steatohepatitis and fibrosis of liver, using the NAFLD activity score and fibrosis stage.
Of the 52 patients, 3 patients in each group (placebo and liraglutide) missed end of treatment biopsies, and one in placebo group dropped out by not taking medications. Baseline age, race and co-morbidities were similar in both groups, with the liraglutide group having more males and lower BMI, though patients in both groups were overweight with BMI’s >30 (liraglutide average BMI 34.2 vs. placebo average BMI 37.7). The average NAFL activity score in the liraglutide group was 4.9 vs. 4.8 in the placebo, with slightly higher steatosis scores in the liraglutide group (liraglutide 2.1 vs. placebo 1.9) and higher advanced fibrosis scores F3–F4 in the placebo group (liraglutide 46% vs. placebo 58%) at baseline. Statistical significance of these differences is not stated, though differences in BMI appear significant.
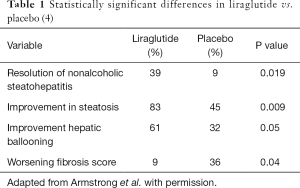
At end of treatment, 39% of the liraglutide group had resolution of steatohepatitis with no worsening of fibrosis vs. 9% of the placebo group which is statistically significant (P=0.019) (Table 1). Other statistically significant results (P≤0.05) include patients with improvement of steatosis (83% in liraglutide group vs. 45% placebo, P=0.009), patients with improvement in hepatocyte ballooning score (61% with liraglutide, 32% with placebo, P=0.05), and worsening fibrosis score with placebo (9% worsened with liraglutide vs. 36% worsened with placebo, P=0.04). No statistically significant change was seen in total NAFLD activity score, lobular inflammation or improvement in fibrosis score. Other statistically significant changes from baseline (liraglutide vs. placebo) were glucose levels (P=0.005), and Hgb A1c (P=0.03), absolute weight (P=0.003), BMI (P=0.005), HDL (P=0.01), GGT levels (P=0.01) enhanced liver fibrosis test (P=0.05), and the physical component of the quality of life questionnaire (SF-36v2 questionnaire, P=0.04). Side effects experienced were mostly mild to moderate and found in both groups; the exceptions were upper and lower gastroenterological side effects which were found more frequently in the liraglutide group.
Comparison to other medications used for NASH
The compelling question when looking at the data for liraglutide is whether the endpoints met are better in liraglutide than other medications that have been studied in the management of NASH. A systemic review and meta-analysis by Singh et al. (5), evaluated the effectiveness of including vitamin E, other TZD, obeticholic acid (OCA) and pentoxifylline in affecting NAFLD score, hepatocyte ballooning, lobular inflammation, steatosis and fibrosis improvement. This analysis only looked at randomized controlled studies with biopsy proven NASH that had a comparator group including placebo, and excluded agents that were known to be ineffective and those that had only short follow up of less than 6 months. This analysis included nine trials with 964 participants with NASH, and found that pentoxifylline and OCA were superior to placebo for improving fibrosis; vitamin E, TZD’s and OCA are superior to placebo for improving hepatocyte ballooning; TZD’s, pentoxifylline and OCA are superior to placebo for improving steatosis and lobular inflammation (see Table 2). However, there is no 5-year follow up on the efficacy of these agents, and limited head to head data. Further studies have also uncovered some possible clinically significant side effects to these agents including possible cardiovascular effects of vitamin E (6) and other TZD’s. Ultimately, there is no significant difference in efficacy of liraglutide compared to other agents (vitamin E, other TZD’s, OCA or pentoxifylline) based on our review.

Full table
Clinical questions: strengths and weaknesses
This phase 2 study has strong design characteristics: double-blinded, randomized and placebo-controlled study with liver biopsy as the baseline and endpoint test to evaluate the effects of liraglutide on NAFLD. It also took variability of liver biopsy into consideration, and utilized two independent and blinded evaluators (pathologists) of baseline and endpoint biopsies. The study had a small number of patients in each group as is characteristic of a phase 2 study, but each group also included some patients with cirrhosis and diabetes mellitus. Control group and placebo groups were well matched with the exception of BMI.
The next issue is the efficacy of liraglutide. In this study, liraglutide study group showed improvement of NAFLD such as steatosis and hepatocyte ballooning but not overall NAFLD activity score. There was also an improvement in weight and glycemic index of participants receiving liraglutide. A phase 3 study would need to evaluate if the improvement of steatosis and no worsening of fibrosis was due to weight loss and improved glycemic control alone or the effect of the drug itself, and if these improvements were sustainable after withdrawal of medication or with post drug weight gain. The lack of improvement of NAFLD activity score and fibrosis score is concerning; if NASH and/or fibrosis do not improve with the use of liraglutide, it cannot be assumed that the essential goal of decreasing mortality can be achieved.
Conclusions
Armstrong et al.’s study (4) is overall a strong phase 2 trial with some limitations in results. The data that we have from this phase 2 study shows no indication that the efficacy of liraglutide will be better than other known agents that are already been studied. That being said, there is of course no long term data or head to head trials with known agents to evaluate that definitively. Ideally, we would like to see an agent for NASH that shows improvement in fibrosis and NAFLD activity score, not just a lack of worsening of these endpoints. Ultimately, we recommend a phase 3 study with a large number or patients and adequate follow up post treatment to highlight these issues.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015;148:547-55. [Crossref] [PubMed]
- Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012;55:2005-23. [Crossref] [PubMed]
- Angulo P. Diagnosing steatohepatitis and predicting liver-related mortality in patients with NAFLD: two distinct concepts. Hepatology 2011;53:1792-4. [Crossref] [PubMed]
- Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016;387:679-90. [Crossref] [PubMed]
- Singh S, Khera R, Allen AM, et al. Comparative effectiveness of pharmacological interventions for nonalcoholic steatohepatitis: A systematic review and network meta-analysis. Hepatology 2015;62:1417-32. [Crossref] [PubMed]
- Dietrich M, Jacques PF, Pencina MJ, et al. Vitamin E supplement use and the incidence of cardiovascular disease and all-cause mortality in the Framingham Heart Study: Does the underlying health status play a role? Atherosclerosis 2009;205:549-53. [Crossref] [PubMed]