Improvement of nutritional support strategies after surgery for benign liver tumor through nutritional risk screening: a prospective, randomized, controlled, single-blind clinical study
Introduction
It has become an increasing popular practice in clinical nutrition that patients were given nutritional risk assessment before surgery as guidance for the preoperative nutritional support program. The previous prospective, randomized studies showed that for the patients suffering from cancer who had obvious nutritional risk (NRS 2002 greater than 3 points), it is beneficial to the patients in postoperative recovery and clinical outcomes if they were provided preoperative nutritional support 3-4 days before surgery (1). However, these studies did not distinguish individual disease; it is difficult to draw conclusion for individual treatment from these studies. These studies had made advance in the field of surgical clinical nutrition, but the results are not without controversy. More studies are needed to obtain more evidence to guide the nutrition support strategy.
Previous studies regarding the nutritional risk assessment on surgical patients were focused on patients with severe disease or cancer. There are very few reports about the preoperative nutritional status evaluation or postoperative nutritional support strategy for patients suffered from benign disease with probably no nutritional risk (2-4). It is not clear that whether the nutritional risk assessment or previously proposed “permissive underfeeding” theory is still applicable in directing the postoperative nutritional fluid composition for patients without nutritional risk. The strategy of postoperative energy support for these patients remains vague (5).
The improvement in liver transplantation along with the adoption of precise liver resection technique has greatly improved the safety of the liver resection surgery in recent years. The surgical trauma for multi-lobe liver resection becomes less serious; there is less blood loss and fewer surgical complications (6). In parallel with these improvements, it is necessary to evaluate the preoperative nutritional risk assessment and the postoperative nutrition support strategy for the patients suffered from benign liver tumor. In this study, we performed nutritional risk assessment for patients of benign tumor before surgery, and randomly divided the patients into two groups for different postoperative nutritional supports. We recorded and compared various postoperative clinical parameters and clinical outcome, and found that for patients with benign liver tumor, it is economically beneficial without compromising the clinical outcome that they receive lower total energy in the postoperative nutrition support.
Materials and methods
Subjects
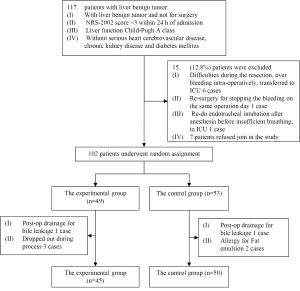
We recruited a total of 95 patients who underwent liver resection for benign tumor during the time period of March 2010 to April 2011 in the Peking Union Medical College Hospital. The study protocol with human subjects was approved by the Ethical Committee of PUMCH and then designed (ClinicalTrials.gov Identifier: NCT01292330) with a research process shown in Figure 1.
Composition of NRS (2002) nutrition risk screening score: (I) damage of nutritional status, including: BMI, recent changes in body weight and food intake (0 to 3 points); (II) disease severity (0 to 3 points): hip fractures, acute or chronic disease complications, COPD, hemodialysis, liver cirrhosis, usually cancer patients (will be assigned as 1), major abdominal surgery, stroke, severe pneumonia, blood cancer (2 points), traumatic brain injury, bone marrow transplant, ICU patients with APACHE score >10 points (3 points); (III) age (≥70 years of age, 1 point) (7).
SIRS score: SIRS diagnosis was given when any two of the following symptoms exist: (I) body temperature is higher than 38 °C or lower than 36 °C; (II) pulse is greater than 90 beats/min; (III) breathing rate is greater than 20 /min or PaCO2 is lower than 32 mmHg; (IV) WBC count is greater than 12×109 /L, or fewer than 4×109 /L, or immature red blood cells consists more than 10% of total red blood cells. Each exist symptom is given a score of 1, and the severity of SIRS range from 0-4.
APACHE-II is consisted of APS, age, and CPS.
Survey methodology
The enrolled surgical patients were randomly divided into two groups: the experimental group (45 patients total, where 39 hepatic hemangioma, 2 FNH, 1 hepatic adenoma, 1 cystadenoma, 1 abnormal nodules and 1 liver cyst) and the control group (50 patients total, where 46 hepatic hemangioma, 2 FNH and 2 angiomyolipoma). Patients in the experimental group were given glucose, electrolyte supplement; patients with heavier weight, diabetes, or greater surgical trauma (such as semi-hepatectomy) were given additional lipid emulsion, the total energy uptake is 42 kJ/kg/d. Patients in the control group were given standard parenteral nutrition supplement in which 30-40% of the total energy uptake of 75 kJ/kg/d consists of the energy from fat. There is no difference in the total volume of iv fluid, additional electrolyte, vitamins, or mineral supplement between the two groups. The volume of iv fluid was gradually reduced at same rate for both groups 3-5 days after surgery when patients started eating.
Clinical observations: blood draw was performed from each patient on the day before and day 1, 3, 5, and 9 after surgery for the following tests including routine blood tests, liver and kidney function, blood coagulation, hemoglobin, alanine aminotransferase, albumin, direct bilirubin, indirect bilirubin, fasting blood glucose level, and prothrombin time. Patients were also observed for time of flatus, infections after surgery, including respiratory, urinary tract, wound, intra-peritoneal, and intravenous catheter infection. Record of wound dehiscence or difficult of healing were taken. The length of hospitalization, total inpatient expenses and nutrition-related expense were also recorded.
The length of hospitalization for each patient is defined as the day before surgery to the day for suture removal. For patient leaving the hospital before stitch removal, the day of discharge is considered the end of hospital stay. If the patient suffers from infection or complications after stitch removal, the length of stay in the hospital for treatment is counted as part of total hospital stay. Nutrition-related cost is defined as the total cost minus the cost of the surgery, cost of special medication, and any cost that is not related to parenteral nutrition related costs. The cost for complication treatment will be included in the total cost. This completed study is registered with Clinicaltrials.gov, number NCT01292330.
The statistical analysis
Data were shown as mean ± standard deviation. Student’s t test or chi-square test was used for group comparison. Liner regression for repeated measure analysis of variance used to compare variables before and after surgery between the two different trends (including WBC, HGB, ALT, ALB, TBIL, DBil, GLU, PT). It was considered statistically significant when P<0.05. SPSS12.0 was used in statistical computing.
Results
General conditions
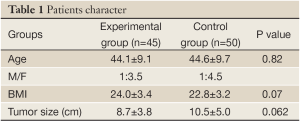
Both groups of patients suffered from benign liver tumors. The average ages of patients in the experimental group and control group are similar (44.2±9.1 vs. 44.6±9.7 years), and there are no significant differences between the groups in male/female ratio (1/3.5 vs. 1/4.5), patient average BMI (24.1±3.5 vs. 22.9±3.3), or average tumor size (8.8±3.8 vs. 10.6±5.0 cm) (Table 1). There are no statistical differences in the following clinical parameters between the groups before surgery, including: WBC, HGB, ALT, ALB, TBil, DBil, fasting blood glucose, and PT. There was no intolerance or adverse reaction among patients in the course of this study; no medications or treatments that would potentially influence the results were involved. All patients were able to complete the clinical studies successfully.
Full table
Patients character
Changes of vital signs
Patients from both groups experienced an increase of their daily high temperature and pulse after surgery before they return to normal levels. The recovery of patients in the experiment group is faster than the patients in control group. The difference of average pulse between the two groups (82.0±12.5 vs. 87.2±9.9 beats/min, P<0.05) on the first day after surgery is statistically significant. So is the difference in the average daily temperature between the groups temperature (37.5±0.3 vs. 37.8±0.5 °C, P<0.05) on the third day after surgery (Table 2).

Full table
The WBC count and hemoglobin level
Hemoglobin levels for patients in both groups declined during the first 5 days after surgery, and subsequently started to recover from day 5. There is no significant difference in the changes of hemoglobin levels between the two groups. The WBC count for patients in both groups increased significantly after surgery, while the counts are highest in the first day after surgery, and then gradually reduced to normal. There is no significant difference in the changes of WBC count between the two groups either (Table 3).
Full table
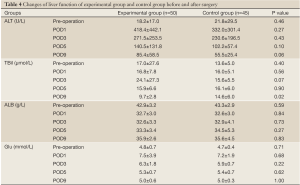
Changes in liver and kidney function
Patients in both groups experienced some decrease in albumin levels in the first 5 days after surgery, which started to rebound in day 5. The serum ALT levels for patients in both groups were elevated substantially in the first 3 days after surgery. However, the differences in the changes of albumin or serum ALT levels between the two groups were not statistically significant. There is significant difference on the total bilirubin levels at day 9 after surgery between the two groups (9.7±2.8 vs. 14.6±6.1 µmol/L, P<0.05). Both groups of patients had normal postoperative renal function. They all had increased fasting blood glucose levels on the first day after surgery, which retreated gradually to normal afterwards (Table 4).

Full table
Post-operative inflammatory reactions
Experimental group and control group after the first day of two groups of APACHE II score (4.78 vs. 4.95, P>0.05).
Complications
There were 2 cases of wound lipid liquefaction and 1 case of bile leakage in the experimental group, and no infectious complications. There were 2 cases of wound lipid liquefaction and 1 case of pulmonary infection in the control group. Patients with complications were successfully treated with frequent change of dressing or anti-biotic regiments. None of the patients in either group had developed intra-abdominal, intravenous catheter infections and urinary tract infections.
The flatus time, the length of hospital stay and treatment related costs
The average flatus time (3.6±0.8 vs. 3.6±0.8 days) and the average length of hospital stay (9.7±1.7 vs. 10.0±2.2 days) for patients in the experimental group and the control group were similar, there were no statistically differences.
The average nutrition-related cost for patients in the experimental group is significantly lower than that for the patients in the experimental group (494.0±181.0 vs. 1,514.4±348.4 RMB, P<0.05). So is the total cost for treatment (18,495.2±4,735.0 vs. 21,432.7±8,291.2 RMB, P<0.05).
Discussion
In 2002, the European Society of Parenteral and Enteral Nutrition published a guideline for nutrition screening of hospitalized patients known as the NRS method, which is established based on the available evidences for the relationships between 128 sets of nutritional support and clinical outcomes (7). NRS 2002 has not only included the nutritional indicators from several nutrition screening tools, but also considered engaging the increased metabolism and nutritional requirements from the diseases into a scoring system that reflecting the severity of the diseases (8). NRS assess the nutritional status through BMI, recent weight change, food uptake and severity of disease; it processes a linear correlation with the traditional nutrition assessment method. The biggest advantage of NRS is its simplicity, that it can be determined through inquiry and simple tests. There is good communications between doctor and patient, and it is well accepted by most of the patients. Systematic analysis of the 128 random controlled trials on the nutritional support and clinical outcomes by Kondrup et al. shows that for patients who were considered having nutritional risk by NRS 2002 assessment, most studies showed that nutritional support effectively improved clinical outcomes, such as: reducing the complications and shorten the length of stay; while for patients who were considered having no nutritional risk, most studies showed that nutritional support was not effective (9). Chen Wei, Jiang Zhu et al. reported in their studies of the risk of malnutrition using NRS that 33.8% of patients had malnutrition risk and needed nutritional support. This number is comparable to the 31% that was reported in the United States by similar studies, suggesting that NRS 2002 can be used in China (10). Later, several clinical researches on NRS 2002 also showed that NRS 2002 could be widely used on hospitalized patients, and that it had better sensitivity and specificity than some other screening tools. These results further confirmed the relationship between the NRS2002 nutritional risk assessment and the clinical outcomes (11,12).
The purpose of perioperative nutritional support is to maintain the functions of the organs, tissues, and immune system, to promote the repair of the damaged organ or tissue, and to accelerate the rehabilitation of the patients. It generally plays a positive and significant role. However, inappropriate nutritional support will not benefit but harm the patients (13,14). Therefore, more researches are needed to determine the appropriate time, methods, and the nutritional formulations for the perioperative nutritional support to improve the clinical outcomes (15-17).
For most surgical patients who do not possess nutritional risk through nutritional screening and assessment, a safe, reasonable treatment program should be established which consists of simple glucose electrolyte fluid regime. It is not necessary to maintain a nutritional support that is wasteful and can also be potentially risky (18). Long and Chen et al. recommended that ESPEN NRS 2002 method of nutrition risk screening could be applied multiple times during the hospital stay in conjunction with the consideration of the severity of the surgical trauma, operation time, and the postoperative conditions. For patients with no nutritional risk (NRS points <3), there is no need for additional nutritional support, and glucose electrolyte infusion should be sufficient (19). It is still debatable whether water (sodium) should be supplemented or limited during operation. There is still a lack of guidance for the perioperative infusion of glucose and electrolytes, or enough evidence for nutritional support. However, it is agreed that it’s important to the fluid supplement is needed to avoid early postoperative hypovolemia (14,15).
There are not many researches focusing on developing perioperative nutrional support based on preoperative nutritional risk assessment in benign tumor patients with low nutritional risk. Even fewer studies were on the impact of nutritional risk assessment on the clinical outcomes in a single disease. Thus, evidence-based prospective, randomized, controlled clinical study can be of great importance for the clinical practice (20,21). In the present study, we summarized the observations of 95 cases of benign liver tumors. We designed the total energy uptake in our experimental group, which is 42 kJ/kg/d, based on 60% of the total energy intake for the permissive underfeeding. Our results showed that, although the energy intake for the experimental group was significantly lower than that of the control group, there is no significant differences on hemoglobin and WBC count on day 1, 3, and 5 after surgery. The low-calorie supplement did not change the hemoglobin and white blood cells reactions after surgery; it did not affect the serum ALT levels or changes in albumin and blood sugar during recovery. The patients in the experimental group recovered faster than those in the control group in terms of body temperature, pulse and total bilirubin levels. There are no differences between the two groups in the flatus time or length of hospitalization, however, the nutrition-related costs and the total cost of hospitalization for the experimental-group patients was significantly lower than those of the control group. We believe that in the situation that both groups had similar surgical trauma and postoperative recovery, patients in the control group consumed more calories, resulting in increased medical costs.
Our results suggested that in the era of precise liver resection, patient who underwent hepatic resection of liver segment for benign liver tumors, could adopt nutritional support program that provided lower energy than the limit of the permissive underfeeding program, if the patient’s preoperative NRS score less than 3 points. This lower energy support program resembles more to the scale for the short-term, mild, systemic inflammatory response syndrome (SIRS) patients. Further study is needed for the mechanism of the lower energy support. However, the following point of view is receiving increasing attention in the academic field, that is, when severe systematic inflammatory response is not a risk, the patient’s natural metabolism should be left undisrupted by the energy supplement to be the main source in providing the energy needed for recovery.
Conclusions
Patient with benign liver tumors can adopt an even lower postoperative nutritional supply that close to that for mild non-surgical conditions, and lower than the postoperative permissive underfeeding standard.
Acknowledgements
This research was supported by the China Medical Board of New York (CMB) grant 06-837.
Disclosure: The authors declare no conflict of interest.
References
- Johansen N, Kondrup J, Plum LM, et al. Effect of nutritional support on clinical outcome in patients at nutritional risk. Clin Nutr 2004;23:539-50.
- Martindale RG, McClave SA, Vanek VW, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition: Executive Summary. Crit Care Med 2009;37:1757-61.
- Doig GS, Simpson F, Finfer S, et al. Effect of evidence-based feeding guidelines on mortality of critically ill adults: a cluster randomized controlled trial. JAMA 2008;300:2731-41.
- Li J. The trend of the development of clinical nutrition support. Parenteral & Enteral Nutrition 2010;17:1-4.
- Mao Y, Lu X, Sang X, et al. Permissive underfeeding in post-operative patients: results of a prospective, randomized, controlled clinical trial. Chin J General Surg 2005;20:612-5.
- Clavien PA, Petrowsky H, DeOliveira ML, et al. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med 2007;356:1545-59.
- Kondrup J, Allison SP, Elia M, et al. ESPEN guidelines for nutrition screening 2002. Clin Nutr 2003;22:415-21.
- Corish CA, Flood P, Kennedy NP. Comparison of nutritional risk screening tools in patients on admission to hospital. J Hum Nutr Diet 2004;17:133-9; quiz 141-3.
- Kondrup J, Rasmussen HH, Hamberg O, et al. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr 2003;22:321-36.
- Jiang ZM, Chen W, Zhan WH, et al. Parentteral and enteral nutrition application in China: A survey in 19 hospitals of 13 major metropolitan cities with 15098 patients by using the Nutrition Risk Screening (2002).Clin Nutr 2007;suppl 2:133-4.
- Li N. Surgical perioperative problems perioperative nutritional support. J Clin Surg 2006;14:544-5.
- Finucane TE. Evidence-based nutrition guidelines for critically ill adults. JAMA 2009;301:1543; author reply 1543-4.
- Li J. Thinking of individual Nutrition therapy in critically ill patients. Parenteral & Enteral Nutrition 2009;16:193-4.
- Chen Y. Effects of trauma and infection stresses on protein metabolism. Parenteral & Enteral Nutrition 2010;17;172-4,178.
- Jacob M, Chappell D, Rehm M. Clinical update: perioperative fluid management. Lancet 2007;369:1984-6.
- Ziegler TR. Parenteral nutrition in the critically ill patient. N Engl J Med 2009;361:1088-97.
- Bozzetti F, Forbes A. The ESPEN clinical practice Guidelines on Parenteral Nutrition: present status and perspectives for future research. Clin Nutr 2009;28:359-64.
- Amaral TF, Matos LC, Tavares MM, et al. The economic impact of disease-related malnutrition at hospital admission. Clin Nutr 2007;26:778-84.
- Dong G, Chen Y, Jiang Z. Evidence-based utilization of nutrition support or glucose & electrolytes nutritional support in the elderly surgical patients. J Clin Surg 2008;16:795-6.
- Zander R. Perioperative fluid management. Anaesthesist 2006;55:1113-4; author reply 1114-6.
- Martins CP, Correia JR, do Amaral TF. Undernutrition risk screening and length of stay of hospitalized elderly. J Nutr Elder 2005;25:5-21.