Immune surveillance of liver cancer in non-alcoholic fatty liver disease: excess lipids cause CD4 T-cells loss and promote hepatocellular carcinoma development
Non-alcoholic fatty liver disease (NAFLD) is the most common liver disease in industrialized countries with an increasing prevalence worldwide (1). Large-scale epidemiological studies have further associated NAFLD with various metabolic risk factors, mainly obesity and diabetes, and these conditions are clearly rising worldwide (2). Work of the last decades has shown that innate immune cells and inflammatory pathways play a central role in the pathogenesis of NAFLD (3). Patients with active inflammatory NAFLD, termed non-alcoholic steatohepatitis (NASH), are at particular risk for progressing to liver cirrhosis. Importantly, patients with NASH can develop hepatocellular carcinoma (HCC), even in non-cirrhotic livers (4).
There have been great research efforts to identify the molecular and cellular mechanisms linking NAFLD and inflammation to HCC development. Patients with NASH as well as mouse models of the disease revealed a prominent activation of the innate immune system, especially macrophages. On the one hand, liver macrophages propagate hepatic inflammation and fibrosis; on the other hand, they might contribute to a pro-tumorigenic microenvironment via secretion of angiogenic factors and suppressing T-cells (5). During the process of ongoing hepatic fat accumulation, not only innate but also adaptive immune cells are attracted to migrate into the liver. They interact with liver tissue cells, become activated by various lipids and other metabolic stress factors and promote liver damage and HCC development (6). Overall, these data support the view of HCC being an “inflammation-driven” cancer (7).
More recently, adaptive immune cells were recognized in liver cancer for their tumor surveillance function, assigning them an important anti-tumoral role (8,9). However, the precise molecular mechanisms of adaptive immune cell activation in HCC, especially in the context of a steatotic liver, have been largely unknown. In a recent hallmark paper that was published by the group of Tim Greten, the dysregulation of lipid metabolism in NAFLD was described to damage tumor-suppressive CD4 T-cells, which causes a selective loss of intrahepatic anti-tumoral CD4 T lymphocytes (10).
In this paper, the authors made use of one of the first mouse models of HCC that conditionally expresses a tetracycline regulatory MYC transgene preferentially in liver cells (11). It allows the induction of tumours that resemble human HCC. These transgenic mice were used to investigate how metabolic changes that occur during the pathogenesis of NAFLD might promote hepatocarcinogenesis (10). When respective animals with activated MYC protein were fed with a methionine-choline deficient (MCD) diet, the mice developed earlier liver tumours than respective controls. Similar findings were observed when mice were fed with a choline-deficient and amino acid-defined (CDAA) diet. A synergism between tumorigenic stimuli and NAFLD in the formation of HCC was also found in diethylnitrosamine carcinogen-challenged wild type mice that were fed with a CDAA or a high-fat diet (10).
Interestingly, in all tested models, the authors found that the number of conventional intrahepatic CD4+ T lymphocytes was selectively reduced during diet-induced NAFLD. This observation appears of fundamental importance. CD4+ T-cells have a suppressive activity on tumour formation by induction of cellular senescence (12), shutdown of angiogenesis, and expression of chemokines/cytokines that contribute to the remodeling of the microenvironment required for sustained tumor regression (9,13). Therefore, the authors concluded that the selective loss of intraheptic CD4+ T lymphocytes might be causative involved in the progression from NAFLD to HCC (Figure 1).
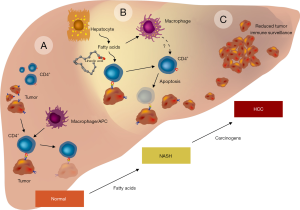
To prove this hypothesis, the authors performed selective depletion of intrahepatic CD4+ T lymphocytes using an anti-CD4 antibody in wild-type or in mice overexpressing the MYC oncoprotein that were fed with a MCD diet. In both experiments, CD4 depletion significantly promoted HCC development. In the presence of lipid-laden hepatocytes isolated from NASH mice, CD4+ T-cells but not CD8+ T-cells underwent apoptosis. Moreover, further experiments showed that the impact of hepatocytes on CD4+ T-cells does not require cell-to-cell contact, suggesting the release of lipid-related factors from hepatocytes in NAFLD. In fact, similar findings were seen when the CD4+ T-cells were stimulated with free fatty acids (FFA), while the depletion of FFA no longer caused CD4+ T lymphocyte death (10). From the different FFA that are most prominent in lipid-laden hepatocytes, the efficacy of linoleic acid (C18:2) in stimulating CD4+ T lymphocyte cell death was found to be the strongest. In CD4+ but not in CD8+ lymphocytes, this fatty acid also significantly induced oxidative phosphorylation, mitochondrial dysfunction and impaired electron transport complex function that was combined with a significant decrease in mitochondrial membrane potential. As a consequence, cell death occurred due to formation of premature electron leakage to oxygen and generation of higher levels of mitochondrially-derived reactive oxygen species (ROS). In accordance, the authors found that ROS scavengers such as N-acetylcysteine or enzymes that protect against oxidative damage by decomposition of hydrogen peroxide such as catalase are therapeutically effective in preventing C18:2-induced CD4+ T lymphocyte death in vitro and in vivo. Moreover, these ROS antagonizing strategies significantly reduced NAFLD-associated tumour development (10). Based on their experimental findings, the authors speculate that the observed NAFLD-associated loss of CD4+ lymphocytes is a key factor that contributes to HCC. To further demonstrate that all these findings are also relevant to human disease, the authors showed that C18.2 also effectively induced selective CD4+ human lymphocyte death. In addition, patients suffering from NASH and alcoholic steatohepatitis (ASH) had markedly reduced hepatic CD4+ T lymphocyte counts as compared to patients with viral hepatitis (10).
Undoubtedly, the study presents a wealth of data and applied a high diversity of sophisticated methods. However, there are still open intriguing questions that are worth to be confirmed in other models or addressed by future studies. For example, the myc oncogene that was used as an HCC formation stimulus is itself critically involved in the formation of hepatic steatosis. It for example stimulates the expression of the carbohydrate responsive element-binding protein (ChREBP) (14). The liver-specific inhibition of ChREBP improves hepatic steatosis (15). ChREBP represents a basic helix-loop-helix leucine zipper transcription factor that controls transcription of lipogenic enzyme genes and is a central regulator of glycolysis and de novo fatty acid synthesis in the liver, thereby playing an important role in the development of diabetes, obesity, and hypertension (16). It might therefore be possible that the overexpression of MYC in the MYC-ON mice when fed a MCD diet might provoke additional effects related to the effects of MYC in steatogenesis.
The authors identified linoleic acid, one of the fatty acids significantly accumulating in NAFLD, as a causative agent triggering CD4+ T-cells apoptosis. Co-stimulation experiments showed that this polyunsaturated omega-6 fatty acid is released from lipid-laden hepatocytes causing mitochondrial ROS formation, oxidative damage and selective loss of CD4+ T lymphocytes (10). The finding that linoleic acid is a strong inducer of apoptosis is not new. It is well-accepted that short-chain fatty acids are not toxic even at high concentrations, while longer, more unsaturated fatty acids and volatile fatty acids can already cause cell death, apoptosis and necrosis in low concentrations (17). In addition, a previous study has shown that the linoleic acid-induced cell death involves mitochondrial depolarization, intracellular lipid accumulation, overexpression of p53 and c-myc, and ROS production in a human immortalized T lymphocyte cell line (Jurkat) and in primary human peripheral blood mononuclear cells (18,19). Similarly, also cells of hepatocyte origin are prone to linoleic acid. In rat hepatoma cell line H4IIE, the application of linoleic acid induces endoplasmic reticulum stress and apoptosis, while the co-treatment of linoleic acid and palmatic acid had no impact on apoptosis (20). Interestingly, the depletion of intracellular calcium flux by a calcium-specific chelator abolished the apoptosis-inducing activity of linoleic acid in this set of experiments. Therefore, it would be of fundamental importance to test in future studies, which effects are obtained when CD4+ cells are triggered with combinations of fatty acids and if calcium blockers can overcome the tremendous effects of linoleic acid in CD4+ lymphocytes. Moreover, it is advisable to further dissect effects on different subsets of CD4+ T-cells, given the partially counteracting functions of Th1, Th2, Th17 or regulatory T-cells as well as other CD4-expressing lymphocytes such as natural killer T (NKT) or innate lymphoid cells (ILC) in the liver (5). Along this line, lipids do not only impact T-cells directly, but tremendously modulate the ability of dendritic cells (DC) to present antigens, which is a pre-requisite for effective T-cells responses (21).
The observed differences of CD4+ and CD8+ T lymphocytes towards linoleic acid may have also implication for other diseases. Obese people are more likely than people of normal weight to develop infections (22). In particular, a recent study that evaluated the association between infections and obesity in patients with end-stage liver disease showed that obesity is independently associated with bacteraemia and tissue infection (23). Based on the data presented by Ma and colleagues, it is possible that the high content of linoleic acid impacts systemic or hepatic CD4 and CD8 T-cells counts or changes the CD4/CD8 ratio that can be anticipated to alter immune responses to infectious agents. Interestingly, in line with their CD4+ depletion hypothesis, the authors found fewer hepatic CD4+ cells in NASH and ASH patients. However, older lymphocyte immunophenotyping studies in liver tissue showing high ratios of CD4/CD8 in patients suffering from fatty liver malignancies (24,25). Interestingly, a recent study that enrolled 112 patients suffering from different fat-associated hepatic diseases and 43 healthy controls reported that NAFLD patients show that the progression of NAFLD to NASH is marked by an increased frequency of IL-17 positive cells among intrahepatic CD4+ T lymphocytes (26).
In summary, the study by the Greten group identified a novel pathogenic link between obesity-induced lipid accumulation and a selective CD4+ T-cells loss in liver tissue. The authors suggest that the depletion of this cell entity is the major cause impairing tumor immunity and enhancing HCC development during NAFLD progression. It will be interesting to see in the current ongoing clinical trials whether patients with NAFLD-associated HCC will have a similar response to novel immunotherapeutic treatments such as administration of immune checkpoint inhibitors (e.g., anti-PD-1, anti-PD-L1, anti-CTLA-4) or whether the proposed lipid-mediated selective CD4+ T-cells loss might render them refractory to this emerging therapeutic option (27).
Acknowledgements
Both authors are supported by the German Research Foundation (SFB/TRR 57). The authors thank Sabine Weiskirchen for preparing the figure.
Footnote
Provenance: This is a Guest Editorial commissioned by Editor-in-Chief Yilei Mao (Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Beijing, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Do A, Lim JK. Epidemiology of nonalcoholic fatty liver disease: a primer. Clin Liver Dis 2016;7:106-8. [Crossref]
- NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 2016;387:1377-96. [Crossref] [PubMed]
- Arrese M, Cabrera D, Kalergis AM, et al. Innate immunity and inflammation in NAFLD/NASH. Dig Dis Sci 2016;61:1294-303. [Crossref] [PubMed]
- Zoller H, Tilg H. Nonalcoholic fatty liver disease and hepatocellular carcinoma. Metabolism 2016;65:1151-60. [Crossref] [PubMed]
- Heymann F, Tacke F. Immunology in the liver--from homeostasis to disease. Nat Rev Gastroenterol Hepatol 2016;13:88-110. [Crossref] [PubMed]
- Wolf MJ, Adili A, Piotrowitz K, et al. Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell 2014;26:549-64. [Crossref] [PubMed]
- Mossanen JC, Tacke F. Role of lymphocytes in liver cancer. Oncoimmunology 2013;2:e26468. [Crossref] [PubMed]
- Schneider C, Teufel A, Yevsa T, Staib F, Hohmeyer A, Walenda G, Zimmermann HW, Vucur M, Huss S, Gassler N, Wasmuth HE, Lira SA, et al. Adaptive immunity suppresses formation and progression of diethylnitrosamine-induced liver cancer. Gut 2012;61:1733-43. [Crossref] [PubMed]
- Kang TW, Yevsa T, Woller N, et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature 2011;479:547-51. [Crossref] [PubMed]
- Ma C, Kesarwala AH, Eggert T, et al. NAFLD causes selective CD4(+) T lymphocyte loss and promotes hepatocarcinogenesis. Nature 2016;531:253-7. [Crossref] [PubMed]
- Felsher DW, Bishop JM. Reversible tumorigenesis by MYC in hematopoietic lineages. Mol Cell 1999;4:199-207. [Crossref] [PubMed]
- Braumüller H, Wieder T, Brenner E, et al. T-helper-1-cell cytokines drive cancer into senescence. Nature 2013;494:361-5. [Crossref] [PubMed]
- Rakhra K, Bachireddy P, Zabuawala T, et al. CD4(+) T cells contribute to the remodeling of the microenvironment required for sustained tumor regression upon oncogene inactivation. Cancer Cell 2010;18:485-98. [Crossref] [PubMed]
- Zhang P, Metukuri MR, Bindom SM, et al. c-Myc is required for the CHREBP-dependent activation of glucose-responsive genes. Mol Endocrinol 2010;24:1274-86. [Crossref] [PubMed]
- Dentin R, Benhamed F, Hainault I, et al. Liver-specific inhibition of ChREBP improves hepatic steatosis and insulin resistance in ob/ob mice. Diabetes 2006;55:2159-70. [Crossref] [PubMed]
- Filhoulaud G, Guilmeau S, Dentin R, et al. Novel insights into ChREBP regulation and function. Trends Endocrinol Metab 2013;24:257-68. [Crossref] [PubMed]
- Lima TM, Kanunfre CC, Pompéia C, et al. Ranking the toxicity of fatty acids on Jurkat and Raji cells by flow cytometric analysis. Toxicol In Vitro 2002;16:741-7. [Crossref] [PubMed]
- Cury-Boaventura MF, Pompéia C, Curi R. Comparative toxicity of oleic acid and linoleic acid on Jurkat cells. Clin Nutr 2004;23:721-32. [Crossref] [PubMed]
- Cury-Boaventura MF, Gorjão R, de Lima TM, et al. Comparative toxicity of oleic and linoleic acid on human lymphocytes. Life Sci 2006;78:1448-56. [Crossref] [PubMed]
- Zhang Y, Xue R, Zhang Z, et al. Palmitic and linoleic acids induce ER stress and apoptosis in hepatoma cells. Lipids Health Dis 2012;11:1. [Crossref] [PubMed]
- Herber DL, Cao W, Nefedova Y, et al. Lipid accumulation and dendritic cell dysfunction in cancer. Nat Med 2010;16:880-6. [Crossref] [PubMed]
- Falagas ME, Kompoti M. Obesity and infection. Lancet Infect Dis 2006;6:438-46. [Crossref] [PubMed]
- Sundaram V, Kaung A, Rajaram A, et al. Obesity is independently associated with infection in hospitalised patients with end-stage liver disease. Aliment Pharmacol Ther 2015;42:1271-80. [Crossref] [PubMed]
- Li XM, Jeffers LJ, Reddy KR, et al. Immunophenotyping of lymphocytes in liver tissue of patients with chronic liver diseases by flow cytometry. Hepatology 1991;14:121-7. [Crossref] [PubMed]
- Chiou YL, Shih CJ, Ko WS. The increased ratio of CD4+/CD8+ was positively correlated with inflammation in hepatitis C patients with metabolic syndrome. Clin Biochem 2013;46:745-9. [Crossref] [PubMed]
- Rau M, Schilling AK, Meertens J, et al. Progression from nonalcoholic fatty liver to nonalcoholic steatohepatitis is marked by a higher frequency of Th17 cells in the liver and an increased Th17/resting regulatory T cell ratio in peripheral blood and in the liver. J Immunol 2016;196:97-105. [Crossref] [PubMed]
- Hato T, Goyal L, Greten TF, et al. Immune checkpoint blockade in hepatocellular carcinoma: current progress and future directions. Hepatology 2014;60:1776-82. [Crossref] [PubMed]


