Association of primary tumor lymph node ratio with burden of liver metastases and survival in stage IV colorectal cancer
Introduction
Colorectal cancer (CRC) is the third most common cancer in the United States (1). The most important prognostic factor is the TNM stage and up to 60% of patients with CRC will develop metastases during the course of their disease (2). Outcomes in these patients depend heavily on the nature and extent of distant metastases (3). The significance of primary tumor characteristics, such as lymph node (LN) status on survival in patients with Stage II/III CRC is well established (4,5). The implications of primary tumor draining LN burden are considered less pivotal in the setting of stage IV disease.
National guidelines recommend evaluation of at least 12 LNs for adequate staging in CRC (6). The extent of nodal involvement is a well established prognostic factor in patients with non-metastatic CRC (4,5,7). In addition to conventional LN status, several authors have proposed surrogate methods of nodal assessment for more accurate staging (8,9). Of those, a valuable tool is the lymph node ratio (LNR), defined as the ratio of positive LNs to the total number of nodes harvested. Recently, the significance of LNR has been explored for various neoplasms including CRC. Although some authors have suggested a role for LNR in stage IV CRC (9,10), the relative dearth of such evidence limits its utility in planning multimodality management of these patients.
The majority of patients with stage IV CRC have liver dominant metastatic disease. Outcomes in such patients are defined by the extent of hepatic tumor burden (3,11). Although aggressive primary tumor characteristics portend a high risk for distant metastases (10,11), the correlation between primary tumor biology and the extent of intrahepatic metastatic disease requires further clarification. The biologic significance of nodal staging in patients with liver metastases (LM) may become increasingly important as patients with synchronous disease are more likely to obtain control of their liver disease with more effective systemic regimens and potentially immunotherapy (12). As such, patients who present with synchronous LM are likely to undergo primary tumor resection at some point during the course of their disease (13).
The objective of our study was to assess the impact of primary tumor LNR in stage IV CRC on survival and its association with the extent of hepatic tumor burden. Such associations would confirm the importance of rigorous nodal staging during resection of the primary tumor in patients with disseminated disease. We hypothesized that high primary tumor LNR is associated with more extensive hepatic metastases and worse survival outcomes in stage IV CRC patients. To our knowledge, an association between primary tumor LNR and intrahepatic tumor burden has not been previously reported.
Methods
With approval of the Institutional Review Board and in accordance with Health Insurance Portability and Accountability Act regulations, a prospectively maintained Roger Williams Cancer Center tumor database was used. Between 2004 and 2011, 79 patients with stage IV CRC were treated at our institution. We excluded 26 patients who did not undergo resection of the primary tumor. Retrospective chart review was then performed on the remaining 53 patients that met final inclusion criteria. Variables examined included age, gender, primary tumor site, LN status, burden of hepatic metastases, carcinoembryonic antigen (CEA) level, presence of extra-hepatic metastases and surgical intervention.
Odds ratio (OR) calculations were used for quantitative assessment of associations and statistical analysis was derived using the chi-square test. A multiple regression model was utilized to determine multivariate statistical independence on factors that were found to be significant on univariate comparison. LNR was defined as the ratio of primary tumor LN with metastatic carcinoma to the total number of LN retrieved. With the assumption of a normal distribution of data, all the associated variables were tested on a correlation matrix using the Pearson product-moment correlation test. Correlation coefficient was calculated by linear regression. LNR cut-off values were analyzed for predictability using receiver operating characteristic (ROC) curves. Survival curves were generated using the Kaplan-Meier (KM) method and statistical comparison was done using the log-rank test. The cox regression model was used for multivariate survival analysis. All statistical analyses report 95% confidence intervals (CI) and were performed using SPSS for windows (SPSS Inc, Chicago, IL, USA). Significance of difference was assumed at P<0.05.
The decision and timing of surgical resection of primary tumor and LM was based on clinical assessment by a surgical oncologist in conjunction with a multidisciplinary team. Extent and nature of hepatic surgery was similarly individualized for each patient based on rigorous clinicopathologic evaluation. Patients were selected for hepatic resection when complete tumor clearance was possible along with an anticipated adequate liver remnant.
Results
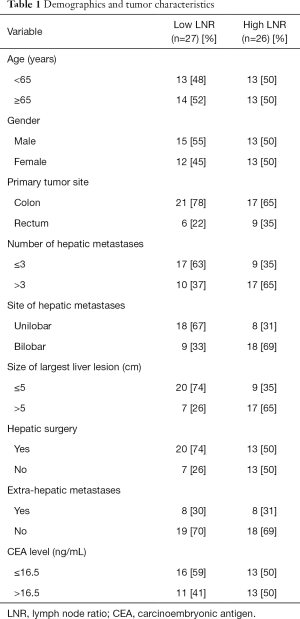
The median number of LNs retrieved was 17 (range, 2–39). Thirty-nine patients (74%) had ≥12 LNs retrieved, consistent with national guidelines. Median follow up was 16 months (range, 0–117 months). The median LNR was 0.25 (range, 0–0.94) and used a cutoff to define patients with a low LNR (L-LNR, ≤0.25) or high LNR (H-LNR, >0.25), as previously reported (8). Among 53 eligible patients, 26 (49%) had H-LNR. Demographic and tumor characteristics with respect to LNR status are outlined in Table 1.
Full table
Age and gender were fairly well distributed within both groups. Most primary tumors were of colonic origin (72%), while the remaining were rectal adenocarcinomas. The median CEA level was 16.5 ng/mL (range, 0.5–2,725 ng/mL). This value was used as a cutoff for comparative analysis. Among all patients, 51% had >3 LM, 51% had bilobar hepatic involvement and 45% had a hepatic metastasis >5 cm. Sixteen patients (30%) had extrahepatic metastases, with lung being the most common site of disease outside the liver. Fourteen patients with extrahepatic metastases (n=16, 88%) did not undergo any hepatic surgery. All patients received chemotherapy either in the neo-adjuvant or adjuvant setting. The dose and timing of chemotherapy was individualized to each patient after a multidisciplinary discussion. Fifteen patients with rectal cancer (n=15, 100%) and 12 patients with colon cancer (n=38, 32%) received neo-adjuvant chemotherapy prior to resection of the primary tumor.
LNR analysis
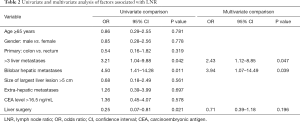
We did not identify an association between age, gender, primary tumor site or CEA level with LNR (Table 2). We found that H-LNR was associated with the presence of >3 LM (OR: 3.21; 95% CI, 1.04–9.88; P=0.042) and bilobar LM (OR: 4.50; 95% CI, 1.41–14.28; P=0.011). Size >5 cm or the presence of extra-hepatic disease were not significant correlates of LNR. Patients with H-LNR were significantly less likely to undergo surgical resection of LM (OR: 0.25; 95% CI, 0.07–0.81; P=0.021). On multiple regression analysis (Table 2), factors independently associated with LNR included the presence of >3 LM (OR: 2.43; 95% CI, 1.12–8.85; P=0.047) and bilobar disease (OR: 3.94, 95% CI, 1.07–14.49; P=0.039). Surgical intervention for metastatic disease was negatively correlated with H-LNR however it was not statistically significant (Table 3).
Full table

Full table
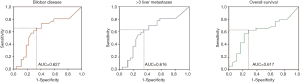
ROC curves were derived to analyze the predictive value of LNR on liver tumor burden and survival (Figure 1). A cutoff of 0.25 was found to be the most predictive for the presence of >3 LM (sensitivity 63%, specificity 65%), bilobar hepatic metastases (sensitivity 65%, specificity 67%) and overall survival (OS) (sensitivity 63%, specificity 72%). Lower LNR cutoffs increased the sensitivity but decreased the specificity of this parameter and the reverse was noted for higher cutoffs.
Survival analysis
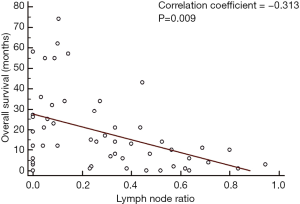
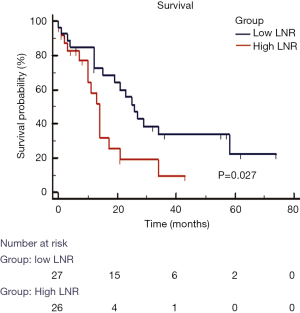
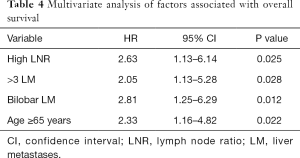
In our group of patients with colorectal LM, with data assumed to be normally distributed, increasing LNR was correlated with worse OS. On linear regression, each increase of LNR value by 0.1 was associated with a decrease in OS by 3.1 months (Figure 2, P=0.009). The median OS for patients with H-LNR was 14 months as compared to 26 months for those with L-LNR (Figure 3). The 3-year OS for H-LNR group was also significantly worse than the L-LNR group (9% vs. 34%, P=0.027). On multivariate analysis of the entire cohort (Table 4), factors independently associated with worse OS included age ≥65 years (HR: 2.33; 95% CI, 1.16–4.82; P=0.022), H-LNR (HR: 2.63; 95% CI, 1.13–6.14; P=0.025), presence of >3 LM (HR: 2.05; 95% CI, 1.13–5.28; P=0.028), and bilobar hepatic metastases (HR: 2.81; 95% CI, 1.25–6.29; P=0.012).
Full table
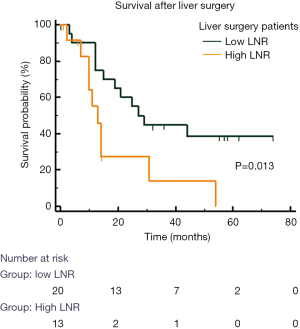
LNR was also associated with worse OS in patients who underwent resection of LM (Figure 4). In these patients, the median OS in H-LNR group was significantly worse than the low LNR group (13 vs. 27 months, P=0.013). The 5-year OS for patients with L-LNR was 37% while none of the patients in the H-LNR group were alive at 5 years following resection of LM. Seven patients in the H-LNR group (27%) and 4 patients in the L-LNR group (15%) had high risk features defined as the presence of bilobar hepatic metastases, >3 LM along with the presence of extra-hepatic disease. The median survival of H-LNR patients with high risk features was 11 months compared to 21 months for those L-LNR group with high risk features (P=0.029).
Discussion
The management of patients with stage IV CRC has evolved considerably over the past decade. Advances in multimodal care have led to improved survival outcomes (14). Although LN status serves as a strong prognostic determinant in CRC patients without distant metastases, the significance of primary tumor LNR is not well understood in the context of stage IV disease. Outcomes in such patients are largely defined by the extent and nature of hematogenous metastatic disease (13). We have demonstrated that primary tumor LNR predicted long-term survival following resection of LM and served as an independent predictor of intrahepatic tumor burden.
Currently, national guidelines recommend retrieval of at least 12 LN for appropriate staging in CRC (6). Nodal stage is determined by the number of positive nodes in the current TNM staging system (AJCC 7th edition), as opposed to LNR. Adequate lymphadenectomy enables appropriate staging of patients, which greatly facilitates management decisions in patients with CRC (15). Although adequate nodal staging should be attempted in all patients, a variety of factors can influence the number of LN ultimately retrieved. Appropriate surgical technique along with diligent pathologic examination of resected specimen are required to consistently attain that goal (16,17). Due to variability in surgical technique and pathologic assessment of specimens, recent studies have proposed alternative LN parameters which may be more reliable than standard nodal staging in predicting outcomes (8,9). The most thoroughly investigated alternative nodal staging approach is LNR (18,19).
The prognostic significance of LNR has been well established for several solid organ malignancies (20,21), including CRC (22). Few studies have evaluated the role of LNR in stage IV CRC. Derwinger and Gustavsson (10) reported worse survival outcomes with increasing LNR in patients with stage IV CRC. However, in that study, the median number of nodes assessed was 10, whereas it is 17 in our present report. Furthermore, the authors did not correlate LNR with extent of intrahepatic metastatic disease. Ozawa et al. (23) recently reported a relationship between LNR and survival in patients with metastatic CRC. In our patients, the median survival of stage IV CRC patients was 26 months in the L-LNR group and 14 months in the H-LNR group. Similarly, for patients with high risk features or those who had undergone surgical intervention for CRLM, the 5-year OS was significantly better in the L-LNR group.
Vaccaro et al. (24) showed that LNR >0.25 was an independent prognostic factor for overall and cancer specific survival in patients with non-metastatic CRC. Several other LNR cutoffs have been proposed based on quartiles, means and various other statistical derivations without any reliable consensus (25,26). We used the median LNR as the cutoff as reported by Ozawa and colleagues (23). On ROC analysis, this cutoff value was found to be the most accurate in predicting hepatic tumor burden and survival in our patients. Importantly, we confirmed in our study group that LNR correlated with survival time in a continuous manner, indicating that our results were not dependent on a particular LNR value.
The majority of patients with stage IV CRC present with liver-only or liver-dominant metastatic disease. All of the patients included in our study had LM and 30% had extra-hepatic disease as well. Surgical resection of CRC LM is the preferred approach (27,28) but unfortunately many patients who are diagnosed with metastatic disease are found to be unresectable at the time of initial evaluation (29). Pathological characteristics of the primary tumor, including LNR, predict the extent of metastatic disease and may assist in identifying high risk patients. In our study, LNR was associated with the presence of more than 3 LM and bilobar disease. Both of these factors weigh heavily when a patient is considered for liver surgery (30). This association may be used to identify patients undergoing liver surgery who are at high risk for recurrence and potentially benefit from neoadjuvant therapy or require more intensive surveillance.
Due to the retrospective design, the exact influence of specific confounding variables could not be quantified. We speculate that the longevity in survival seen in patients with low LNR was influenced by favorable tumor biology in general. While adjuvant therapy likely impacted the outcome of patients in our study, our small sample size did not allow us to examine this in stratified fashion. However, the association of survival with LNR remained significant on multivariate model. Due to improvements in multimodality care, patients with LM from CRC are experiencing prolonged periods of disease control. Primary tumor biologic surrogates, such as LNR, may acquire increased relevance in patients with LM who survive for extended periods of time.
Conclusions
With the development of novel therapeutic options, patients with stage IV CRC have improved outcomes. Risk stratification of these patients may further assist in improvement of care and potentially open avenues for further progress. Considering LNR for patients with metastatic CRC who have undergone surgical resection of the primary tumor should be considered a routine parameter in the evaluation of these patients. Our study defines the potential utility of LNR in predicting hepatic tumor burden and survival, which may influence the complex decisions surrounding multimodality management of patients with stage IV CRC. We believe that even in the setting of stage IV disease, if resection of the primary tumor is required, principles of appropriate nodal staging should be practiced.
Acknowledgements
We would like to acknowledge the contribution of Cheryl Raffel, cancer tumor registrar, for assisting in data acquisition.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by institutional ethics board of Roger Williams Medical Center (No. 00000088888) and written informed consent was obtained from all patients.
References
- National Cancer Institute. Surveillance, Epidemiology, and End Results Program. SEER Stat Fact Sheets: Colon and Rectum Cancer. Accessed: Dec 5, 2015. Available online: http://seer.cancer.gov/statfacts/html/colorect.html
- Van Cutsem E, Nordlinger B, Adam R, et al. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur J Cancer 2006;42:2212-21. [Crossref] [PubMed]
- Miller G, Biernacki P, Kemeny NE, et al. Outcomes after resection of synchronous or metachronous hepatic and pulmonary colorectal metastases. J Am Coll Surg 2007;205:231-8. [Crossref] [PubMed]
- Le Voyer TE, Sigurdson ER, Hanlon AL, et al. Colon cancer survival is associated with increasing number of lymph nodes analyzed: a secondary survey of intergroup trial INT-0089. J Clin Oncol 2003;21:2912-9. [Crossref] [PubMed]
- Gunderson LL, Jessup JM, Sargent DJ, et al. Revised TN categorization for colon cancer based on national survival outcomes data. J Clin Oncol 2010;28:264-71. [Crossref] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Version 2.2016. Available online: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf
- Johnson PM, Porter GA, Ricciardi R, et al. Increasing negative lymph node count is independently associated with improved long-term survival in stage IIIB and IIIC colon cancer. J Clin Oncol 2006;24:3570-5. [Crossref] [PubMed]
- Ozawa T, Ishihara S, Sunami E, et al. Log odds of positive lymph nodes as a prognostic indicator in stage IV colorectal cancer patients undergoing curative resection. J Surg Oncol 2015;111:465-71. [Crossref] [PubMed]
- Peschaud F, Benoist S, Julié C, et al. The ratio of metastatic to examined lymph nodes is a powerful independent prognostic factor in rectal cancer. Ann Surg 2008;248:1067-73. [Crossref] [PubMed]
- Derwinger K, Gustavsson B. A study of lymph node ratio in stage IV colorectal cancer. World J Surg Oncol 2008;6:127. [Crossref] [PubMed]
- Rosen SA, Buell JF, Yoshida A, et al. Initial presentation with stage IV colorectal cancer: how aggressive should we be? Arch Surg 2000;135:530-4; discussion 534-5. [Crossref] [PubMed]
- Katz SC, Burga RA, McCormack E, et al. Phase I Hepatic Immunotherapy for Metastases Study of Intra-Arterial Chimeric Antigen Receptor-Modified T-cell Therapy for CEA+ Liver Metastases. Clin Cancer Res 2015;21:3149-59. [Crossref] [PubMed]
- Ruo L, Gougoutas C, Paty PB, et al. Elective bowel resection for incurable stage IV colorectal cancer: prognostic variables for asymptomatic patients. J Am Coll Surg 2003;196:722-8. [Crossref] [PubMed]
- Khoo CK, Vickery CJ, Forsyth N, et al. A prospective randomized controlled trial of multimodal perioperative management protocol in patients undergoing elective colorectal resection for cancer. Ann Surg 2007;245:867-72. [Crossref] [PubMed]
- Chang GJ, Rodriguez-Bigas MA, Skibber JM, et al. Lymph node evaluation and survival after curative resection of colon cancer: systematic review. J Natl Cancer Inst 2007;99:433-41. [Crossref] [PubMed]
- Prandi M, Lionetto R, Bini A, et al. Prognostic evaluation of stage B colon cancer patients is improved by an adequate lymphadenectomy: results of a secondary analysis of a large scale adjuvant trial. Ann Surg 2002;235:458-63. [Crossref] [PubMed]
- Huh JW, Kim CH, Kim HR, et al. Factors predicting oncologic outcomes in patients with fewer than 12 lymph nodes retrieved after curative resection for colon cancer. J Surg Oncol 2012;105:125-9. [Crossref] [PubMed]
- Ng M, Roy-Chowdhury S, Lum SS, et al. The impact of the ratio of positive to total lymph nodes examined and outcome in colorectal cancer. Am Surg 2009;75:873-6. [PubMed]
- Wang J, Hassett JM, Dayton MT, et al. Lymph node ratio: role in the staging of node-positive colon cancer. Ann Surg Oncol 2008;15:1600-8. [Crossref] [PubMed]
- Bando E, Yonemura Y, Taniguchi K, et al. Outcome of ratio of lymph node metastasis in gastric carcinoma. Ann Surg Oncol 2002;9:775-84. [Crossref] [PubMed]
- Voordeckers M, Vinh-Hung V, Van de Steene J, et al. The lymph node ratio as prognostic factor in node-positive breast cancer. Radiother Oncol 2004;70:225-30. [Crossref] [PubMed]
- Berger AC, Sigurdson ER, LeVoyer T, et al. Colon cancer survival is associated with decreasing ratio of metastatic to examined lymph nodes. J Clin Oncol 2005;23:8706-12. [Crossref] [PubMed]
- Ozawa T, Ishihara S, Nishikawa T, et al. Prognostic significance of the lymph node ratio in stage IV colorectal cancer patients who have undergone curative resection. Ann Surg Oncol 2015;22:1513-9. [Crossref] [PubMed]
- Vaccaro CA, Im V, Rossi GL, et al. Lymph node ratio as prognosis factor for colon cancer treated by colorectal surgeons. Dis Colon Rectum 2009;52:1244-50. [Crossref] [PubMed]
- Rosenberg R, Engel J, Bruns C, et al. The prognostic value of lymph node ratio in a population-based collective of colorectal cancer patients. Ann Surg 2010;251:1070-8. [Crossref] [PubMed]
- Sabbagh C, Mauvais F, Cosse C, et al. A lymph node ratio of 10% is predictive of survival in stage III colon cancer: a French regional study. Int Surg 2014;99:344-53. [Crossref] [PubMed]
- Nordlinger B, Guiguet M, Vaillant JC, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Française de Chirurgie. Cancer 1996;77:1254-62. [Crossref] [PubMed]
- Scheele J, Stangl R, Altendorf-Hofmann A. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg 1990;77:1241-6. [Crossref] [PubMed]
- Jones RP, Vauthey JN, Adam R, et al. Effect of specialist decision-making on treatment strategies for colorectal liver metastases. Br J Surg 2012;99:1263-9. [Crossref] [PubMed]
- Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999;230:309-18; discussion 318-21. [Crossref] [PubMed]


