Neoadjuvant therapy prior to surgical resection for previously explored pancreatic cancer patients is associated with improved survival
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer related deaths in the United States. Optimal treatment of PDAC consists of surgical resection of early stage disease (1,2). Unfortunately, the majority of patients have locally advanced or metastatic disease and only 10–20% are surgical candidates at presentation (1,3-6). Moreover, in patients with “resectable” tumors, the literature suggests that as many as 23–30% are actually found to be unresectable at the time of exploration (3,7,8).
For periampullary carcinoma, the rates of successful resection of patients previously deemed to have unresectable disease are reportedly between 42–100%, and reoperative pancreaticoduodenectomy can be performed with morbidity and mortality similar to patients undergoing primary surgery (9-14). In addition, patients undergoing reoperation for periampullary carcinoma have been reported to have similar long-term survival rates as patients undergoing initial resection (13). Indeed, several small series have demonstrated that re-exploration and successful resection of pancreatic cancer may be achievable in 55–81% of patients initially deemed unresectable (14-17).
The aim of this study was to evaluate the association of neoadjuvant therapy prior to re-exploration on pathologic findings and clinical outcomes in previously explored patients with PDAC. Since the primary reason for aborted resection was an anticipated R2 resection margin, we also compared outcomes of re-explored patients to those patients who had an R2 resection margin at our institution during the same time period.
Methods
Study design and participants
Upon Johns Hopkins Hospital (JHH) Institutional Review Board approval, we queried our prospectively maintained database to identify all patients with PDAC who underwent re-exploratory surgery at JHH following an initial attempt at resection between August 1995 and June 2013. All patients who underwent a prior attempted resection for PDAC and were later successfully resected at JHH were included. Clinical, pathological, surgical and neoadjuvant therapy data were analyzed. We compared pathologic findings and clinical outcomes in these re-explored patients to primarily resected patients with an R2 margin status. Additionally, we compared patients receiving neoadjuvant therapy prior to reexploration to those who were reexplored without neoadjuvant therapy. The median follow up of the re-explored, neoadjuvant plus reexploration and R2 resection group was 17 [interquartile ranges (IQR) 9–37], 25 (IQR 16–50), and 10 (IQR 5–16.5) months, respectively.
Surgical procedures
All 50 patients underwent a re-staging which included a pancreas protocol CT. We defined resectable pancreatic cancer as technically removable tumors with an anticipated negative pathologic margin. Patients with distant metastases, non-reconstructible superior mesenteric or portal vein occlusion, greater than 180 degrees superior mesenteric artery involvement, or encasement of other major vascular structures (celiac axis, hepatic artery, aorta, or inferior vena cava) were excluded. Patients underwent one of the following: a classic pancreaticoduodenectomy, pylorus-preserving pancreaticoduodenectomy, total pancreatectomy, or distal pancreatectomy with splenectomy. Surgical margins were considered microscopically positive (R1) if carcinoma was found within 1 mm of the final resection margin. R2 resection was defined as macroscopically identifiable tumor remnants. Lymph node ratio (LNR) was calculated as the ratio of positive lymph nodes to total lymph nodes removed and then stratified into four groups: 0, 0–0.2, 0.2–0.4, >0.4 (7,18). Pathology was staged according to the American Joint Committee on Cancer (AJCC) Cancer Staging Manual (7th Edition).
Neoadjuvant therapy
Perioperative chemotherapy and radiation data were obtained for the 50 patients from chart review and cancer center registry. Neoadjuvant therapy refers to therapy after first failed exploration and before definitive resection. Treatment consisted of chemotherapy with or without radiation at JHH or other institutions under the care of referring oncologists. There was no standardized neoadjuvant regimen but most patients received gemcitabine-based chemotherapy.
Length of stay (LOS), mortality and survival time
LOS was calculated from date of operation to date of hospital discharge. Ninety-day mortality was defined as any death within 90 days of operation. Morbidity was characterized retrospectively through chart evaluation performed by participating study surgeons. Survival was determined by review of clinic notes, cancer center abstracting services, and the Social Security Death Index. The limitations placed on the Social Security Death Index in March of 2014 which eliminates public access to the Social Security death master file did not preferentially affect any of the groups since review of clinic notes and cancer center abstracting services were also utilized to determine survival. Overall survival since the first operation was calculated from the time of surgery to death. In addition, overall survival from the 2nd resection was calculated from the time of surgery to death.
Statistical analyses
The quantitative parameters of age, interval between surgeries, LOS, operative time, estimated blood loss (EBL), tumor size, positive nodes and total nodes harvested were presented as medians and IQR. Categorical variables between groups were compared by Chi-squared test or Fisher exact test. Continuous variables between groups were compared by Student’s t-test or the Mann-Whitney U test. Survival curves were constructed by the Kaplan-Meier method, and differences in survival were evaluated with a log-rank analysis. The proportion of individuals surviving 1, 2 and 5 years was calculated using life tables. Two-sided P values were always computed and a P value of <0.05 was regarded as statistically significant. All statistical analyses were performed using a commercially available software package (Statistical Package for Social Sciences, SPSS, version 16.0, Chicago, IL, USA).
Results
Demographics
A total of 2,062 pancreatic resections were performed for PDAC at JHH between 1995 and 2013 of which 50 (2.42%) were performed following a previously failed attempt. The characteristics of the 50 re-explored patients and primarily R2 resected locally advanced pancreatic cancer patients are listed in Table 1. The initial exploratory surgery occurred at another institution for 43 patients (86%) and at JHH for 7 patients (14%). The re-explored group included 25 male and 25 female patients, with a median age of 66 (IQR 59.8–75.3) years (Table 1).
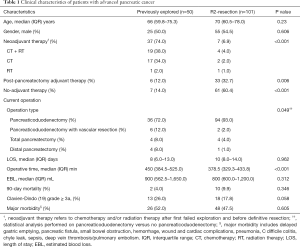
Full table
Initial operation
At the initial operation, 47 (94%) of the 50 patients underwent exploratory laparotomy and 3 (6%) underwent exploratory laparoscopy. The predominant reason cited for unresectability was vascular invasion (80%), of which 9 (22.5%) were arterial and 29 (72.5%) were venous. There were 2 (5%) patients with both arterial and venous involvement cited. The second most common reason for unresectability was celiac or portal lymphadenopathy (10%). The median interval between the initial operation and repeat operation was 154 (IQR 97.8–244.8) days (Table 2).
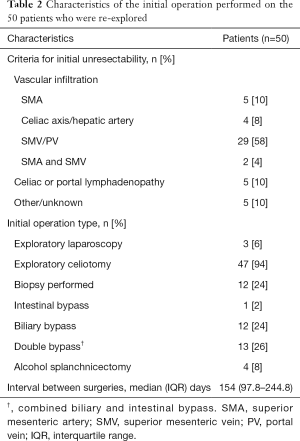
Full table
Resection operative characteristics
Of the 50 re-explored patients, 36 (72%) underwent pancreaticoduodenectomy, 6 (12%) underwent pancreaticoduodenectomy with vascular resection, 4 (8%) underwent total pancreatectomy, and 4 (8%) underwent distal pancreatectomy and splenectomy. Clinical characteristics and surgical outcomes are summarized in Table 1. The perioperative mortality and the overall morbidity rates were 4% and 52% respectively.
Pathology
Histopathology revealed that T3 was the most common T stage for re-explored patients (64%) and the median pathologic tumor diameter was 2.5 cm. Forty-six percent had microvascular invasion and 64% had perineural invasion. The median total number of lymph nodes harvested was 16 and the median number of positive nodes was 0 (Table 3).
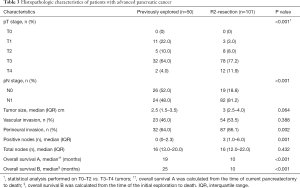
Full table
Administration of neoadjuvant therapy and pathology
Of the 50 re-explored patients, 13 (26%) patients were deemed to be potentially resectable after multidisciplinary assessment which included reimaging at our institution. These patients proceeded to resection rather than neoadjuvant therapy. The other 37 (74%) patients received neoadjuvant therapy. Of these 37 re-explored patients, 19 (38%) patients received chemotherapy and radiation therapy (Table 1). Chemotherapy consisted of both gemcitabine (n=24, 66.7%) and 5-fluorouracil based regimens (n=12, 33.3%).
The administration of neoadjuvant therapy was associated with an increased R0 resection rate (91.9% vs. 61.5%, P=0.016) for 50 patients who were re-explored. The only R2 resections (n=3) occurred in patients who did not receive neoadjuvant therapy. In re-explored patients, those who received neoadjuvant therapy had significantly lower pathologic T and N stages compared with those who did not receive neoadjuvant therapy (P=0.039 and P=0.002, respectively). Neoadjuvant therapy was associated with significantly less microvascular invasion (35.1% vs. 76.9%, P=0.009) and fewer positive lymph nodes (median 0 vs. 2, P=0.025). Treatment with neoadjuvant therapy was associated with more node negative resections (64.9% vs. 15.4%, P=0.002) and lower lymph node ratios (P=0.002) (Table 4).
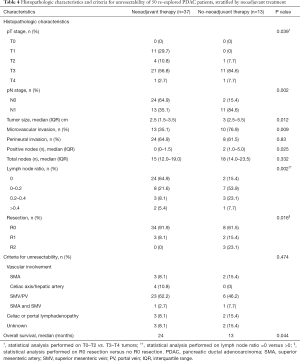
Full table
Survival analysis
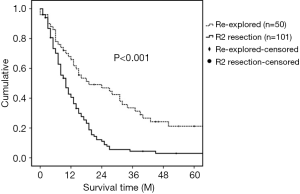
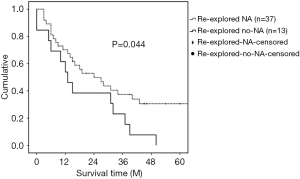
The median survival for patients who underwent resection after a previous exploration was 19 months (Figure 1). Median survival of the resected patients who received neoadjuvant therapy before re-exploration (median 24 months; 95% CI 10.6–37.4) was longer than the median survival for those who did not receive neoadjuvant therapy (median 13 months; 95% CI 7.1–18.9) (Log rank: P=0.044) (Figure 2).
Re-exploration and resection versus R2 resections
We compared the results of 50 previously explored patients who underwent resection to 101 patients that underwent resection at first exploration and had an R2 resection at our institution during the same time period. The main reason for an R2 resection was superior mesenteric artery involvement. Compared with the re-explored group, there were no significant differences in age and gender. Significantly more patients in the re-explored group received neoadjuvant therapy compared to the R2 resection group (74% vs. 6.9% P<0.001) (Table 1). Ninety-four (93%) patients underwent pancreaticoduodenectomy, 2 (2%) patients underwent pancreaticoduodenectomy with vascular resection, 4 (4%) patients underwent total pancreatectomy, and 1 (1%) patient underwent distal pancreatectomy. There was no difference in EBL, LOS, 90-day mortality, or overall morbidity between the two groups (P>0.05), however the previously explored operations were significantly longer (450 vs. 378.5 min, P<0.001) (Table 1).
Re-explored patients had significantly less perineural invasion (64% vs. 86.1%, P=0.002) as well as significantly lower pathologic T and N stages compared to R2 resection patients (both P<0.001) (Table 3). The median survival for patients who underwent re-exploration and resection was significantly greater than R2 resection patients (19 months, 95% CI 3.5–34.5 vs. 10 months, 95% CI 7.6–12.4) (Log rank: P<0.001). The estimated overall survival rates of all re-explored patients were 65.8% at 1 year and 33.5% at 3 years vs. 40.6% at 1 year and 4.4% at 3 years in the R2 resection group (Figure 1).
Discussion
With the advance of high-resolution computed tomography imaging, pre-operative determination of pancreatic cancer resectability has dramatically improved. Pancreatectomy can be aborted if the operator feels it is unsafe or if achieving a negative pathologic margin seems unlikely. Some previously explored patients are treated with neoadjuvant chemotherapy ± radiation therapy and are later resected at a 2nd operation. The outcome of this subgroup is not well described. In this study, we demonstrate improved outcomes for patients who had their pancreatic cancer resected during a 2nd laparotomy compared with patients who had an R2 resection at initial operation during the same time period. We also demonstrate improved pathologic findings and survival associated with the administration of neoadjuvant therapy between operations.
Large series of resected pancreas cancer have reported that the morbidity and mortality are approximately 33–53% and 2–4.4%, respectively (20-27). Our study demonstrates that the overall morbidity and mortality rates are similar between the re-explored group and R2 resection patients (P=0.605 and P=0.35, respectively). However, operative times were longer for re-explorations compared to R2 resections (450 vs. 378.5 min, P<0.001). This difference is likely due to more technically challenging operations in re-explored patients which require more extensive dissection for postoperative adhesions and loss of normal tissue planes. Despite this, operative blood loss and length of hospitalization were not different between the two groups (900 vs. 800 mL, P=0.312; 8 vs. 10 days, P=0.962). Therefore, re-exploration of previously deemed unresectable PDAC can be performed safely.
Neoadjuvant treatment in the form of chemotherapy and/or radiation therapy is being used more frequently to downstage locally advanced unresectable lesions (28-30). Studies evaluating the impact of neoadjuvant chemo radiation have demonstrated that secondary resection becomes possible in about 30–40% of patients with locally advanced disease (3,6). Although no randomized trial has confirmed that margin negative resection rate is increased by neoadjuvant therapy, a group of retrospective studies suggest that neoadjuvant therapy offers the potential of tumor down staging, increasing the likelihood of complete resection with negative surgical margins (29). Furthermore, neoadjuvant therapy theoretically may reduce lymph node metastasis and vascular invasion, preventing peritoneal tumor cell implantation and dissemination during surgery (31,32), subsequently leading to improved survival. Similar to these studies, we demonstrated that re-explored patients treated with neoadjuvant therapy had lower T and N stages (P=0.003 and P=0.002, respectively), less nodal disease rates (15.4% vs. 64.9%; P=0.002), decreased microvascular invasion rates (35.1% vs. 76.9%; P=0.009), increased margin negative resection rates (91.9% vs. 61.5%; P=0.016), and was associated with improved overall survival (24 vs. 13 months; P=0.044).
The prognosis for PDAC correlates with margin status, lymph node metastasis, perineural invasion and perivascular infiltration (33-38). Complete (R0) surgical resection of the tumor with negative lymph nodes is the most important predictor of long-term survival for patients with a potentially resectable PDAC (34,39). In this study, most of the re-explored patients resulted in R0 resections (84%). The median survival of re-explored patients was significantly greater compared to patients resected on the first exploration who had an R2 resection margin (19 vs. 10 months; P<0.001). Our results suggest that if an R2 resection is anticipated, aborting surgery to proceed with neoadjuvant therapy is reasonable.
This single center retrospective study has several limitations. Selection bias is inevitable for this group of patients who underwent re-exploration. The median overall survival in previously explored patients was 19 months from the time of actual resection. Adding the median time between two operations, the median overall survival from initial operation was really 25 months. Currently, median overall survival for resected PDAC is approximately 18.1 months (20). The perceived benefit in overall survival of re-explored patients was very likely impacted by selection bias. In addition, not all patients with PDAC respond to neoadjuvant chemotherapy. Up to 70% of patients with borderline resectable disease will not be resected after neoadjuvant therapy (3,6). In the literature, the median survival of patients who receive neoadjuvant therapy and are never resected is 5–11 months (3). Unfortunately, we do not know how many patients were treated with chemo ± radiation therapy at our institution after aborted resections who progressed and never made it to resection.
The results of our study demonstrate that re-operation for PDAC is safe and effective. The use of neoadjuvant therapy for previously explored patients prior to resection appears to be associated with improved pathology and survival and therefore should be considered for all patients that have been explored previously and deemed locally unresectable.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Johns Hopkins Hospital (JHH) Institutional Review Board (NA_00074221).
References
- Mayo SC, Nathan H, Cameron JL, et al. Conditional survival in patients with pancreatic ductal adenocarcinoma resected with curative intent. Cancer 2012;118:2674-81. [Crossref] [PubMed]
- Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg 2000;4:567-79. [Crossref] [PubMed]
- Gillen S, Schuster T, Meyer Zum Büschenfelde C, et al. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med 2010;7:e1000267. [Crossref] [PubMed]
- Varadhachary GR, Tamm EP, Abbruzzese JL, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol 2006;13:1035-46. [Crossref] [PubMed]
- Callery MP, Chang KJ, Fishman EK, et al. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol 2009;16:1727-33. [Crossref] [PubMed]
- Werner J, Combs SE, Springfeld C, et al. Advanced-stage pancreatic cancer: therapy options. Nat Rev Clin Oncol 2013;10:323-33. [Crossref] [PubMed]
- Epelboym I, DiNorcia J, Winner M, et al. Neoadjuvant therapy and vascular resection during pancreaticoduodenectomy: shifting the survival curve for patients with locally advanced pancreatic cancer. World J Surg 2014;38:1184-95. [Crossref] [PubMed]
- Toomey P, Childs C, Luberice K, et al. Nontherapeutic celiotomy incidence is not affected by volume of pancreaticoduodenectomy for pancreatic adenocarcinoma. Am Surg 2013;79:781-5. [PubMed]
- Hashimi H, Sabanathan S. Second look operation in managing carcinoma of the pancreas and periampullary region. Surg Gynecol Obstet 1989;168:224-6. [PubMed]
- McGuire GE, Pitt HA, Lillemoe KD, et al. Reoperative surgery for periampullary adenocarcinoma. Arch Surg 1991;126:1205-10; discussion 1210-2. [Crossref] [PubMed]
- Robinson EK, Lee JE, Lowy AM, et al. Reoperative pancreaticoduodenectomy for periampullary carcinoma. Am J Surg 1996;172:432-7; discussion 437-8. [Crossref] [PubMed]
- Shukla PJ, Qureshi SS, Shrikhande SV, et al. Reoperative pancreaticoduodenectomy for periampullary carcinoma. ANZ J Surg 2005;75:520-3. [Crossref] [PubMed]
- Sohn TA, Lillemoe KD, Cameron JL, et al. Reexploration for periampullary carcinoma: resectability, perioperative results, pathology, and long-term outcome. Ann Surg 1999;229:393-400. [Crossref] [PubMed]
- Truty MJ, Thomas RM, Katz MH, et al. Multimodality therapy offers a chance for cure in patients with pancreatic adenocarcinoma deemed unresectable at first operative exploration. J Am Coll Surg 2012;215:41-51; discussion 51-2. [Crossref] [PubMed]
- Michalski CW, Kleeff J, Bachmann J, et al. Second-look operation for unresectable pancreatic ductal adenocarcinoma at a high-volume center. Ann Surg Oncol 2008;15:186-92. [Crossref] [PubMed]
- Chao C, Hoffman JP, Ross EA, et al. Pancreatic carcinoma deemed unresectable at exploration may be resected for cure: an institutional experience. Am Surg 2000;66:378-85; discussion 386. [PubMed]
- Johnstone PA, Sindelar WF. Radical reoperation for advanced pancreatic carcinoma. J Surg Oncol 1996;61:7-11; discussion 11-3. [Crossref] [PubMed]
- Pawlik TM, Gleisner AL, Cameron JL, et al. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery 2007;141:610-8. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Wu W, He J, Cameron JL, et al. The impact of postoperative complications on the administration of adjuvant therapy following pancreaticoduodenectomy for adenocarcinoma. Ann Surg Oncol 2014;21:2873-81. [Crossref] [PubMed]
- Nathan H, Cameron JL, Goodwin CR, et al. Risk factors for pancreatic leak after distal pancreatectomy. Ann Surg 2009;250:277-81. [Crossref] [PubMed]
- Yeo CJ, Cameron JL, Sohn TA, et al. Pancreaticoduodenectomy with or without extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma: comparison of morbidity and mortality and short-term outcome. Ann Surg 1999;229:613-22; discussion 622-4. [Crossref] [PubMed]
- Yeo CJ, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg 2002;236:355-66; discussion 366-8. [Crossref] [PubMed]
- Riall TS, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma--part 3: update on 5-year survival. J Gastrointest Surg 2005;9:1191-204; discussion 1204-6. [Crossref] [PubMed]
- He J, Ahuja N, Makary MA, et al. 2564 resected periampullary adenocarcinomas at a single institution: trends over three decades. HPB (Oxford) 2014;16:83-90. [Crossref] [PubMed]
- Mayo SC, Gilson MM, Herman JM, et al. Management of patients with pancreatic adenocarcinoma: national trends in patient selection, operative management, and use of adjuvant therapy. J Am Coll Surg 2012;214:33-45. [Crossref] [PubMed]
- Sabater L, García-Granero A, Escrig-Sos J, et al. Outcome quality standards in pancreatic oncologic surgery. Ann Surg Oncol 2014;21:1138-46. [Crossref] [PubMed]
- Strobel O, Berens V, Hinz U, et al. Resection after neoadjuvant therapy for locally advanced, "unresectable" pancreatic cancer. Surgery 2012;152:S33-42. [Crossref] [PubMed]
- Morganti AG, Massaccesi M, La Torre G, et al. A systematic review of resectability and survival after concurrent chemoradiation in primarily unresectable pancreatic cancer. Ann Surg Oncol 2010;17:194-205. [Crossref] [PubMed]
- Assifi MM, Lu X, Eibl G, et al. Neoadjuvant therapy in pancreatic adenocarcinoma: a meta-analysis of phase II trials. Surgery 2011;150:466-73. [Crossref] [PubMed]
- Zimmermann C, Folprecht G, Zips D, et al. Neoadjuvant therapy in patients with pancreatic cancer: a disappointing therapeutic approach? Cancers (Basel) 2011;3:2286-301. [Crossref] [PubMed]
- Greer SE, Pipas JM, Sutton JE, et al. Effect of neoadjuvant therapy on local recurrence after resection of pancreatic adenocarcinoma. J Am Coll Surg 2008;206:451-7. [Crossref] [PubMed]
- Hartwig W, Hackert T, Hinz U, et al. Pancreatic cancer surgery in the new millennium: better prediction of outcome. Ann Surg 2011;254:311-9. [Crossref] [PubMed]
- Wagner M, Redaelli C, Lietz M, et al. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg 2004;91:586-94. [Crossref] [PubMed]
- Chen JW, Bhandari M, Astill DS, et al. Predicting patient survival after pancreaticoduodenectomy for malignancy: histopathological criteria based on perineural infiltration and lymphovascular invasion. HPB (Oxford) 2010;12:101-8. [Crossref] [PubMed]
- Konstantinidis IT, Warshaw AL, Allen JN, et al. Pancreatic ductal adenocarcinoma: is there a survival difference for R1 resections versus locally advanced unresectable tumors? What is a "true" R0 resection? Ann Surg 2013;257:731-6. [Crossref] [PubMed]
- de Jong MC, Li F, Cameron JL, et al. Re-evaluating the impact of tumor size on survival following pancreaticoduodenectomy for pancreatic adenocarcinoma. J Surg Oncol 2011;103:656-62. [Crossref] [PubMed]
- Slidell MB, Chang DC, Cameron JL, et al. Impact of total lymph node count and lymph node ratio on staging and survival after pancreatectomy for pancreatic adenocarcinoma: a large, population-based analysis. Ann Surg Oncol 2008;15:165-74. [Crossref] [PubMed]
- Yeo CJ, Cameron JL, Sohn TA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg 1997;226:248-57; discussion 257-60. [Crossref] [PubMed]


