Liver transplantation for hepatocellular carcinoma from living-donor vs. deceased donor
Introduction
Since the landmark report of the Milan criteria by Mazzaferro et al. (1), which demonstrated comparable outcomes of patients with hepatocellular carcinoma (HCC) having a single tumor smaller than 5 cm in diameter or up to three tumors smaller than 3 cm in diameter with no vascular invasion or extra-hepatic disease determined by preoperative imaging studies, deceased-donor liver transplantation (DDLT) has become an established treatment for cirrhotic patients with HCC. In Asian countries, where the number of deceased donor is extremely small, living-donor liver transplantation (LDLT) has been developed as an alternative treatment for end-stage liver diseases, and has become an established treatment for those with HCC (2).
With the accumulation of LDLT cases for patients with HCC, the impact of LDLT (the procedure and the partial graft) on the recurrence of HCC after liver transplantation has become an important topic of debate in comparison with DDLT (3,4). The aim of this review was to encompass the current opinions and clinical reports regarding the differences in outcome, especially the recurrence of HCC, between LDLT and DDLT.
The differences between LDLT and DDLT in terms of tumor recurrence
There are definite differences other than the graft type between LDLT and DDLT, such as a shorter waiting time, good quality graft with normal liver function and shorter ischemic time, and pretransplant treatment optimization for HCC, which might potentially contribute to improve survival in LDLT recipients. While these characteristics seem advantageous for the better control of HCC recurrence, some of these characteristics, on the other hand, may lead to a favorable milieu for tumor progression and recurrence (4).
The hypothesized mechanism in experimental studies for the faster tumor progression and the higher recurrence rates in LDLT is that growth factors and cytokines released during rapid regeneration of the partial grafts from the partial graft might contribute to tumor progression and recurrence (5-8). An ischemic-reperfusion injury facilitated by a small-for-size graft and the rapidly regenerating liver parenchyma in LDLT setting may increase vascular endothelial growth factor expression and angiogenesis, which might accelerate the tumor progression and recurrence.
One of the probable explanations for the higher recurrence rates in LDLT is “fast-tracking” patients into liver transplantation (4,9). In the DDLT setting, patients with biologically aggressive HCC might drop off the waiting list due to tumor progression beyond the criteria during the wait-time, however, owing to the shortened wait time in LDLT program, such patients might not be recognized during such a short wait-time, resulting in a possible higher frequency of HCC recurrence in LDLT. This scenario might account for the higher HCC recurrence in the LDLT setting. Bhangui et al. (10) performed a prospective intention-to-treat study demonstrating that shorter waiting time in LDLT prevented the dropouts but worsened the outcomes of LDLT in patients with advanced HCC when compared with DDLT patients with comparable MELD scores. In contrast, Chao et al. (11) failed to show an association between waitlist time and outcome after DDLT or LDLT for HCC.
Since the grafts from living donors are not limited by restrictions imposed by the organ allocation system, and the indication for LDLT in patients with HCC often depends on institutional or case-by-case considerations, balancing the burden on the donor, the operative risk, and the overall survival benefit for the recipient, the expansion of criteria can be easily done without impairing equitability (2). Thus, many LDLT centers adopt the expansion criteria for LDLT recipients, and the advanced tumor characteristics of LDLT recipients can reasonably explain the higher recurrence rate in the LDLT setting (9). Actually, the majority of Asian transplant centers have established and adopted own extended criteria beyond those of Milan or the University of California, San Francisco (UCSF) (2). According to some early studies, differences in patient tumor characteristics between LDLT and DDLT remain a main reason for the higher recurrence rate in LDLT. Additionally, in the majority of studies comparing LDLT and DDLT for HCC patients, tumor burdens such as the size, number, vascular invasion, and poor differentiation have proved to be independent risk factors for HCC recurrence after liver transplantation, all of which may lead to a rational explanation for the impaired recurrence-free survival of LDLT compared to DDLT (12).
Finally, some authors argue that the technique of LDLT per se goes against the principles of oncologic surgery (13,14). During LDLT, the meticulous dissection and mobilization of the liver might increase the possibility of tumor capsule violation or tumor dissemination through the hepatic veins, thus promoting tumor dissemination. Meticulous preservation of the native vena cava and the bile duct/hepatic artery/portal vein in the hepatic hilum might also increase the risk of leaving the residual tumors.
Studies comparing LDLT and DDLT for HCC patients
Studies comparing the recurrence-free survival after LDLT and DDLT for HCC patients are summarized in Table 1. There were ten single-institutional reports (3,10,13-15,17-19,21,22) and three multi-center reports (16,20,23).
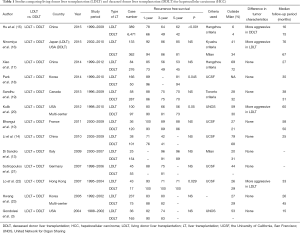
Full table
Park and colleagues (18) from Korea recently reported poorer recurrence-free survival among 166 LDLT recipients (81% at 5 years) compared to 50 DDLT recipients (94% at 5 years; P=0.045). The study was notable in that the smaller the LDLT graft was, the poorer the recurrence-free survival was. They suggested that the physiology of the small graft may stimulate tumor recurrence. The results of the A2ALL cohort in USA (20) demonstrated an impaired outcome in LDLT recipients, in which HCC recurrence remained significantly different between LDLT and DDLT after adjustment for tumor characteristics. They concluded that the higher recurrence observed after LDLT was likely due to differences in the tumor characteristics, pretransplant HCC management, and waiting time between the programs. Lo et al. (22) from Hong Kong also reported a significantly higher incidence of HCC recurrence in LDLT (29%) than in DDLT (0%) (P=0.029). While the tumor characteristics were comparable between the groups, the authors speculated that LDLT in a salvage fashion, higher incidence of microscopic vascular invasion in LDLT, and liver regeneration led to the higher recurrence rate in LDLT.
In contrast, majority of reports have demonstrated the comparable outcomes. Sandhu and colleagues (19) of the Toronto group compared the results of 58 LDLT cases with those of 287 DDLT cases having comparable tumor characteristics, in which the 1-, 3-, and 5-year recurrence-free survival rates were 88%, 75%, and 70%, and 86%, 75%, and 70%, respectively. Bhangui et al. (10) conducted a well-designed intention-to-treat analysis with recurrence rate representing the primary endpoint, comparing 36 LDLT cases and 147 DDLT cases, in which both LDLT and DDLT provided similar recurrence-free survival rates (88% vs. 86% at 5 years). The dropout rate and waiting time were significantly lower in the LDLT group than in the DDLT group, and the time to recurrence in LDLT tended to be longer than in DDLT, which may guarantee additional advantages with LDLT. Similar results were reported by other authors as listed in Table 1.
Systematic review and meta-analysis
A randomized clinical study would be best to settle the controversy regarding the differences in outcomes among LDLT versus DDLT for HCC patients, but this is indeed difficult, given the complicated decision-making process and multidisciplinary approach involved in liver transplantation for HCC patients. No prospective study has ever challenged the matter, however, there were two systematic reviews with meta-analyses so far. Grant et al. (24) performed a meta-analysis on 12 retrospective studies comparing the recurrence rates and recurrence-free survival between LDLT and DDLT recipients. A total of 633 LDLTs and 1,232 DDLTs were enrolled, and the study provided evidence of lower disease-free survival after LDLT compared with DDLT for HCC [hazard ratio =1.59; confidence interval (CI): 1.02−2.49; P=0.041]. In contrast, there was no difference in overall survival between LDLT and DDLT (hazard ratio =0.97; CI: 0.73−1.27; P=0.808). Liang et al. (25) also performed similar meta-analysis based on 7 retrospective studies with a total of 1,310 participants. They found comparable patient survival rates [1 year: odds ratio (OR) =1.03, 95% CI: 0.62−1.73; 3 years: OR =1.07, 95% CI: 0.77−1.48; and 5 years: OR =0.64, 95% CI: 0.33−1.24] and disease-free survival rates (1 year: OR =0.86, 95% CI: 0.54−1.38; 3 years: OR =1.04, 95% CI: 0.69−1.58; and 5 years: OR =1.11, 95% CI: 0.70−1.77). As discussed by both authors of the papers, however, all involved studies were retrospective, had a low data quality score with poor reporting of baseline patient characteristics, and were heterogeneous in critical aspects such as indication criteria and basal tumor characteristics, all which do not give a strong evidence to the results of meta-analyses. In addition, recent US registry study found no significant difference in outcomes between whole and partial grafts among HCC patients (26).
A review article by experts (27) concluded as follows: although there is no strong evidence to support the higher HCC recurrence rates in LDLT than DDLT, the reported higher recurrence rates in LDLT recipients cannot be ignored. In addition to taking into account the differences in organ allocation, graft availability, and organ transplant law and allocation system, liver transplant candidates with HCC and their potential live donors should be informed following risks and benefits; the waiting time for DDLT may lead to the dropout due to HCC progression which could be avoided by the prompt LDLT, however, the prompt LDLT may mask the aggressive tumor characteristics which may lead to a higher HCC recurrence rates. Nevertheless, the tumor characteristics and biology seem to have significant impact on the recurrence, while the graft type and waiting time are less important as a possible risk factor.
Asian perspective regarding liver transplantation for HCC
Milan criteria are also standard indication criteria for liver transplantation for HCC patients in Asian countries. However, in Asia where LDLT is mainstay for liver transplantation, situations are somewhat different from region to region. Unlike DDLT, LDLT is not limited by the restrictions imposed by the nationwide allocation system, and the indication for LDLT in patients with HCC often depends on institutional or case-by-case considerations, balancing the burden on the donor, the operative risk, and the overall survival benefit for the recipient. In Japan, each center developed institutional expansion criteria, while National Insurance covers only those within the Milan criteria (28,29). In Taiwan (30) and Hong Kong (31), UCSF criteria is adopted. In mainland China, Hangzhou or Chengdu criteria is used with a satisfactory outcome (32). In Korea, UCSF or Milan is basically used, but LDLT can be offered for any HCC without distant metastasis under National Insurance coverage (33). In conclusion, Milan criteria are still the gold standard criteria of liver transplantation for HCC patients worldwide, and seem best to be included in the treatment algorithm for HCC to set the tumor burden limitation. Nevertheless, it is widely accepted that the Milan criteria is too strict in terms of post-transplant recurrence rate (34) and that it is currently expanded to some extent without impairing patient outcome in Asian countries (2), however, we have to always be aware of that any kind of expansion in size or number of the tumor includes the potential to worsen the post-transplant survival in patients with HCC.
The indication of liver transplantation for HCC in terms liver functional reserve is based on the model for end-stage liver disease (MELD) score with additional points in Western countries (35). Consequently, liver transplantation can be offered for those with Child class A as shown in guidelines, if they satisfy Milan criteria (36,37). In contrast, in Asian countries, where liver grafts are extremely scarce, liver transplantation is recommended for those with decompensated liver cirrhosis (Child class B and C) in patients with HCC as well as those with other decompensated liver diseases (38).
Conclusions
In conclusion, there is no evidence to support higher HCC recurrence after LDLT than DDLT. LDLT should always be considered as a treatment option for HCC patients with advanced cirrhosis in areas where deceased donors are scarce or for patients whose tumor status interrupts access to grafts from deceased donor.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-9. [Crossref] [PubMed]
- Akamatsu N, Sugawara Y, Kokudo N. Living donor liver transplantation for patients with hepatocellular carcinoma. Liver Cancer 2014;3:108-18. [Crossref] [PubMed]
- Gondolesi GE, Roayaie S, Muñoz L, et al. Adult living donor liver transplantation for patients with hepatocellular carcinoma: extending UNOS priority criteria. Ann Surg 2004;239:142-9. [Crossref] [PubMed]
- Kulik L, Abecassis M. Living donor liver transplantation for hepatocellular carcinoma. Gastroenterology 2004;127:S277-82. [Crossref] [PubMed]
- Yang ZF, Poon RT, Luo Y, et al. Up-regulation of vascular endothelial growth factor (VEGF) in small-for-size liver grafts enhances macrophage activities through VEGF receptor 2-dependent pathway. J Immunol 2004;173:2507-15. [Crossref] [PubMed]
- Man K, Fan ST, Lo CM, et al. Graft injury in relation to graft size in right lobe live donor liver transplantation: a study of hepatic sinusoidal injury in correlation with portal hemodynamics and intragraft gene expression. Ann Surg 2003;237:256-64. [Crossref] [PubMed]
- Shi JH, Huitfeldt HS, Suo ZH, et al. Growth of hepatocellular carcinoma in the regenerating liver. Liver Transpl 2011;17:866-74. [Crossref] [PubMed]
- Efimova EA, Glanemann M, Liu L, et al. Effects of human hepatocyte growth factor on the proliferation of human hepatocytes and hepatocellular carcinoma cell lines. Eur Surg Res 2004;36:300-7. [Crossref] [PubMed]
- Fisher RA, Kulik LM, Freise CE, et al. Hepatocellular carcinoma recurrence and death following living and deceased donor liver transplantation. Am J Transplant 2007;7:1601-8. [Crossref] [PubMed]
- Bhangui P, Vibert E, Majno P, et al. Intention-to-treat analysis of liver transplantation for hepatocellular carcinoma: living versus deceased donor transplantation. Hepatology 2011;53:1570-9. [Crossref] [PubMed]
- Chao SD, Roberts JP, Farr M, et al. Short waitlist time does not adversely impact outcome following liver transplantation for hepatocellular carcinoma. Am J Transplant 2007;7:1594-600. [Crossref] [PubMed]
- Chan SC. Section 2. Small-for-size liver graft and hepatocellular carcinoma recurrence. Transplantation 2014;97:S7-S10. [Crossref] [PubMed]
- Di Sandro S, Slim AO, Giacomoni A, et al. Living donor liver transplantation for hepatocellular carcinoma: long-term results compared with deceased donor liver transplantation. Transplant Proc 2009;41:1283-5. [Crossref] [PubMed]
- Li C, Wen TF, Yan LN, et al. Outcome of hepatocellular carcinoma treated by liver transplantation: comparison of living donor and deceased donor transplantation. Hepatobiliary Pancreat Dis Int 2010;9:366-9. [PubMed]
- Hu Z, Qian Z, Wu J, et al. Clinical outcomes and risk factors of hepatocellular carcinoma treated by liver transplantation: A multi-centre comparison of living donor and deceased donor transplantation. Clin Res Hepatol Gastroenterol 2016;40:315-26. [Crossref] [PubMed]
- Ninomiya M, Shirabe K, Facciuto ME, et al. Comparative study of living and deceased donor liver transplantation as a treatment for hepatocellular carcinoma. J Am Coll Surg 2015;220:297-304.e3. [Crossref] [PubMed]
- Xiao GQ, Song JL, Shen S, et al. Living donor liver transplantation does not increase tumor recurrence of hepatocellular carcinoma compared to deceased donor transplantation. World J Gastroenterol 2014;20:10953-9. [Crossref] [PubMed]
- Park MS, Lee KW, Suh SW, et al. Living-donor liver transplantation associated with higher incidence of hepatocellular carcinoma recurrence than deceased-donor liver transplantation. Transplantation 2014;97:71-7. [Crossref] [PubMed]
- Sandhu L, Sandroussi C, Guba M, et al. Living donor liver transplantation versus deceased donor liver transplantation for hepatocellular carcinoma: comparable survival and recurrence. Liver Transpl 2012;18:315-22. [Crossref] [PubMed]
- Kulik LM, Fisher RA, Rodrigo DR, et al. Outcomes of living and deceased donor liver transplant recipients with hepatocellular carcinoma: results of the A2ALL cohort. Am J Transplant 2012;12:2997-3007. [Crossref] [PubMed]
- Sotiropoulos GC, Lang H, Nadalin S, et al. Liver transplantation for hepatocellular carcinoma: University Hospital Essen experience and metaanalysis of prognostic factors. J Am Coll Surg 2007;205:661-75. [Crossref] [PubMed]
- Lo CM, Fan ST, Liu CL, et al. Living donor versus deceased donor liver transplantation for early irresectable hepatocellular carcinoma. Br J Surg 2007;94:78-86. [Crossref] [PubMed]
- Hwang S, Lee SG, Joh JW, et al. Liver transplantation for adult patients with hepatocellular carcinoma in Korea: comparison between cadaveric donor and living donor liver transplantations. Liver Transpl 2005;11:1265-72. [Crossref] [PubMed]
- Grant RC, Sandhu L, Dixon PR, et al. Living vs. deceased donor liver transplantation for hepatocellular carcinoma: a systematic review and meta-analysis. Clin Transplant 2013;27:140-7. [Crossref] [PubMed]
- Liang W, Wu L, Ling X, et al. Living donor liver transplantation versus deceased donor liver transplantation for hepatocellular carcinoma: a meta-analysis. Liver Transpl 2012;18:1226-36. [Crossref] [PubMed]
- Cauley RP, Potanos K, Fullington N, et al. The effect of graft type on mortality in liver transplantation for hepatocellular carcinoma. Ann Transplant 2015;20:175-85. [Crossref] [PubMed]
- Grant D, Fisher RA, Abecassis M, et al. Should the liver transplant criteria for hepatocellular carcinoma be different for deceased donation and living donation? Liver Transpl 2011;17:S133-8. [Crossref] [PubMed]
- Tamura S, Sugawara Y, Kokudo N. Living donor liver transplantation for hepatocellular carcinoma: the Japanese experience. Oncology 2011;81:111-5. [Crossref] [PubMed]
- Kokudo N, Hasegawa K, Akahane M, et al. Evidence-based clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2013 update (3rd JSH-HCC Guidelines). Hepatol Res 2015.45. [PubMed]
- Chen CL, Concejero AM. Liver transplantation for hepatocellular carcinoma in the world: the Taiwan experience. J Hepatobiliary Pancreat Sci. 2010;17:555-8. [Crossref] [PubMed]
- Yau T, Tang VY, Yao TJ, et al. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology 2014;146:1691-700.e3. [Crossref] [PubMed]
- Xu X, Lu D, Ling Q, et al. Liver transplantation for hepatocellular carcinoma beyond the Milan criteria. Gut 2016;65:1035-41. [Crossref] [PubMed]
- Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology 2016;150:835-53. [Crossref] [PubMed]
- Clavien PA, Lesurtel M, Bossuyt PM, et al. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol 2012;13:e11-22. [Crossref] [PubMed]
- Toso C, Mazzaferro V, Bruix J, et al. Toward a better liver graft allocation that accounts for candidates with and without hepatocellular carcinoma. Am J Transplant 2014;14:2221-7. [Crossref] [PubMed]
- European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908-43. [Crossref] [PubMed]
- Bruix J, Sherman M; Practice Guidelines Committee, Management of hepatocellular carcinoma. Hepatology 2005;42:1208-36. [Crossref] [PubMed]
- Omata M, Lesmana LA, Tateishi R, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int 2010;4:439-74. [Crossref] [PubMed]


