Role of positron emission tomography/computed tomography in living donor liver transplantation for hepatocellular carcinoma
Introduction
Living donor liver transplantation (LDLT) has been established as an effective treatment for hepatocellular carcinoma (HCC) in the era of a critical shortage of deceased donors (1). For selecting appropriate the patients with HCC for liver transplantation (LT), many criteria were suggested based on tumor morphology including size and number (2,3). The representative criteria were the Milan criteria (a solitary tumor no more than 5 cm in diameter, or two or three tumors no more than 3 cm in diameter, no extrahepatic metastasis and major vessel invasion) and the University of California at San Francisco (UCSF), (a solitary tumor no more than 6.5 cm in diameter or two or three tumors with the largest diameter being no more than 4.5 cm and the sum of the diameters being no more than 8 cm, no extrahepatic metastasis and major vessel invasion). In recent, these criteria have been widely accepted to selecting patients with HCC waiting deceased donor LT. However, in Asian countries such as Korea and Japan, the LDLT has become an important option for treatment in patients with HCC, and the amount of experience and evidence on LDLT for HCC has been increased (1,4). The selection criteria for LT have gradually been expanded in large-volume centers, and the good outcome of LDLT for advanced HCC has been reported (5-7).
On the base of these experiences, the expanded criteria using tumor morphology as well as tumor biology have been reported as the Milan criteria using tumor size and number was too restrictive and limited to apply to LDLT. In LDLT setting, although the patients had the advanced HCC beyond the Milan criteria, the operation could be performed as the willingness of donor and informed consent. Therefore, many criteria using various indicators α-fetoprotein (AFP), protein induced by vitamin K absence or antagonist-II (PIVKA-II), 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) and tumor morphology have been proposed (5,8-14). In HCC patients, tumor characteristics, including differentiation grade and microvascular invasion, are well-known independent prognostic factors for overall survival (OS) and disease-free survival (DFS) following LT (15). However, these factors were hard to be evaluated by preoperative imaging tools, which reveal the morphological characteristics such as number and size. Furthermore, some biomarkers including AFP, PIVKA-II, and genomic data had the limitation to predict the tumor pathologic characteristics.
Recently, several studies using 18F-FDG PET/CT in LDLT demonstrated the usefulness of PET/CT to detect the extrahepatic metastases and synchronous malignant neoplasm as well as recurrence of HCC with good performance (5,10-12,16-19). In HCC patients, PET/CT imaging before surgery or transplantation was not popular in worldwide due to cost-effective or technical issues. However, some major centers have continued to report the usefulness of PET/CT in LDLT patients, and so we reviewed PET/CT role in LDLT according to some important aspects.
Role of HCC detection using PET/CT in LDLT
To identify HCC before surgery or transplantation, most common imaging tools were CT, dual-contrast magnetic resonance image (MRI), and ultrasonography (US) (20-24). Recent MRI using hepatobiliary-specific contrast medium such as gadoxetic acid is primarily used to improve detection and characterization of HCC (25,26). This imaging feature can detect very small lesions less than 1 cm and assists in differentiating regenerative/dysplastic nodules from early HCCs with over 90% accuracy.
Recently, whole-body PET/CT usually used 18F-FDG effectively to detect numerous cancerous lesions including HCC (27). However, liver tissue showed the high level of gluoce-6-phosphatase and release the FDG-6-phosphate. This phenomenon demonstrated the reduced discrimination between normal liver tissue and well-differentiated HCC. Thus, 18F-FDG PET/CT showed average false-negative rate of 40–50% for the detection of HCC (28). Teefey et al. reported the comparative results among CT, MRI, US, and PET in liver transplant candidate to detect primary hepatic malignancy (29). They examined 25 patients as liver transplant candidates, and the results were interpreted independently by two radiologists. HCC was diagnosed in nine patients. US diagnostic performance was superior to that of CT and MRI. Sensitivities were higher for US (0.89) than they were for CT (0.67), MRI (0.56), PET (0). Therefore, they concluded that PET did not depict any HCC and was not useful tool for detection of HCC in LT. Some authors used the 11C-labeled acetate PET/CT to detect HCC. 11C-labeled acetate PET/CT effectively detects urologic malignancies (30). This enters the Krebs cycle as a substrate for β-oxidation in fatty acid synthesis and cholesterol synthesis. Fatty acid synthesis is major mechanism for uptake of 11C-acetate by liver tumors. Park et al. showed the detection rate of primary liver tumors using 18F-FDG and 11C-acetate PET/CT in 120 patients (99 with HCC, 13 with cholangiocarcinoma) (31). They resulted that the overall sensitivities of 18F-FDG, 11C-acetate, and dual-tracer PET/CT in the detection of 110 lesions in 90 patients with primary HCC were 60.9%, 75.4%, and 82.7%, respectively. However, the overall sensitivities of 18F-FDG, 11C-acetate, and dual-tracer PET/CT for 35 metastatic HCCs were 85.7%, 77.0%, and 85.7%, respectively. They concluded that the addition of 11C-acetate to 18F-FDG PET/CT increased the overall sensitivity for the detection of primary HCC but not for the detection of extrahepatic metastases. Cheung et al. also reported 11C-acetate and 18F-FDG PET/CT for clinical staging and selection of patients with HCC for LT on the basis of Milan criteria (32). They enrolled the patients with HCC who underwent dual-tracer PET/CT (22 patients in LT, 21 patients in hepatectomy). Dual-tracer PET/CT performed equally well in both LT and partial hepatectomy groups for HCC detection (94.1% and 95.8%) and TNM staging (90.9% and 90.5%). In cirrhotic liver, dual-tracer PET/CT showed better sensitivities for detection of primary tumor than contrast CT. They concluded that the inclusion of dual-tracer PET/CT in pretransplant workup may warrant serious consideration.
However, 11C-acetate PET/CT is not popular in clinical field. PET/CT still has relatively serious limitation to detect HCC within liver compared to other modalities like contrast dynamic CT, liver specific MRI, and US. Therefore, in practice, the use of PET/CT in patient with HCC focused the detection of metastatic HCC and other hidden malignant neoplasm except the liver. In our institute, we started the pretransplant 18F-FDG PET/CT to check other metastatic lesions easily and rule out the hidden other malignancies. We found the several popular malignant neoplasms in Korea, such as thyroid cancer, stomach cancer, and so on. With these experiences, we could apply the PET/CT to other aspects including prognosis, pathology prediction, and criteria.
Predicting pathologic results
Before LT, many efforts to predict the pathology have been performed as the pathologic data are not routinely available. The invasive fine needle biopsy results before LT has the risk of complications including bleeding and does not show any correlations with explant pathology (33). As HCC has microscopic heterogenicity within one nodule, the assessment of pathology is almost impossible until the whole specimen is reviewed. Therefore, during several decades, tumor morphological characteristics including number and size by preoperative imaging modalities were used to predict the aggressiveness of pathology. However, tumor number and size was very limited to predict important pathologic variables including microvascular invasion and differentiation. Recently, biomarkers, response to transarterial chemoembolization (TACE), gene-expression profile, PET/CT, AFP, and PIVKA-II, have been used for the assessment of tumor aggressiveness (5,10-12,34-39). In particular, 18F-FDG PET/CT represented the biologic status of tumor aggressiveness and showed good correlation with explant pathology in HCC (Table 1). For the accuracy of detection of microvascular invasion in PET/CT, the range was 68.3–88.1%. The accuracy of detection of tumor differentiation in PET/CT was 54.7% to 71.4%. In Lee et al.’s study including large number of patients, the sensitivity and specificity of microvascular invasion, differentiation, major vessel invasion, serosal invasion, and intrahepatic metastasis were 40.7%/74.5%, 51.9%/79.9%, 86.4%/72.5%, 61.3%/76.2%, and 43.8%/73.9%, respectively. According to these findings, PET/CT in LT patient with HCC did not show strong correlation in aspect of microvascular invasion and differentiation. This is another limitation not to replace the morphological characteristics to predict the prognosis or explant pathology. However, PET/CT showed the possibility predicting the biological activity and tumor aggressiveness demonstrating the accuracy around 70–90%. This tool is helpful to supplement the disadvantage of prediction with only tumor morphology before LT.
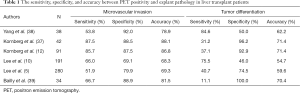
Full table
Predicting tumor recurrence
Beyond the association with explant pathology, PET/CT showed the results highly related to tumor recurrence and patient’s prognosis. The definition of PET/CT positivity was not clear because background liver also demonstrated highly uptake of 18F-FDG. Although experienced nuclear medicine doctor’s reports were important, the value of maximum standardized uptake value (SUVmax), ratio of tumor to normal-liver SUVmax, and uptake-volume products have been objective indices to defined the PET/CT positivity of tumor (16). Among them, SUVmax was popular as a main factor to define the PET/CT positivity. Several studies showed the poor overall and recurrence-free survival in PET/CT positive patients with HCC after LT (Table 2). DFS with PET/CT positive patients showed the various range around 40–50% at 3 years after LDLT. These were poor outcome compared to that with PET/CT negative patients, which showed around 90% at 3 years after LDLT. In particular, the gap in patients beyond the Milan criteria was widen compared to patients within the Milan criteria. Lee et al. showed the difference of 35.8% in patients beyond the Milan criteria according to PET/CT positivity (5). In patients within the Milan criteria, there was the difference of only 16.0% between PET/CT positive and negative patients. Lee et al. suggested that PET/CT findings were more important in patients with advanced HCC. Even though the patients had advanced HCC, it could be an indicator to select the patients with good prognosis as the patients beyond the Milan criteria without PET/CT positivity showed relatively good 5 years DFS with 73.3%. Patients with HCC beyond the Milan criteria with a PET/CT negative status and total tumor size less than 10 cm showed similar OS and DFS in comparison with those with HCC within the Milan criteria. Furthermore, in the analysis of 191 patients with PET/CT after LDLT, 20 patients (10.5%) showed early recurrence (less than 6 months after LDLT). PET/CT positive status was identified as an independent prognostic factor for DFS influencing early recurrence in multivariable analysis (HR =3.945, P=0.024) (10). This means that PET/CT reflects the tumor aggressiveness and early tumor recurrence after LDLT. Furthermore, positive uptake of PET/CT has been observed in poorly differentiated HCC and presence of microvascular invasion. These findings demonstrated that biologic aggressiveness represented by PET/CT is an important factor for predicting early tumor recurrence. In these patients with PET/CT positivity, close follow-up should be needed in postoperative period of LDLT. Kim et al. reported the usefulness of 18F-FDG PET/CT for detecting recurrence of HCC in posttransplant patients (17). Among 93 patients with LDLT, ten patients were recurred. And PET/CT showed 92.9% accuracy to find the extrahepatic metastases over 1 cm including 100% detection rate in bone and the lymph nodes and 60% in the lungs. In spite of limitations for small lesions, they concluded that PET/CT could provide additional information beyond that provide by conventional modalities. Like these results, PET/CT is a very useful tool to predict the prognosis such as early recurrence and DFS and detect the HCC recurrence in extrahepatic lesions. Furthermore, in advanced HCC patients, PET/CT showed the possibility for selecting patients to LDLT with comparable prognosis.

Full table
The possibility of selecting criteria in LDLT
Over past decades, the Milan criteria have been regarded as a well-established tool for assessing the prognosis of HCC for LT. However, the limitations including narrow selection range and inaccurate assessment using preoperative CT imaging modality have been criticized by many researchers and clinicians, especially in LDLT. LDLT has the special situation easy to expand the criteria for HCC. In some centers, they perform the LDLT beyond the Milan criteria over 50% in their total cases (5). These LDLT beyond the Milan criteria have given the important data about the presence of patients showing good prognosis. Especially, PET/CT plays an important role in advanced HCC patients. As simple morphologic characteristics have the limitation to reflect the tumor aggressiveness correctly, the assessment of biologic activity by PET/CT could represent the chance of tumor recurrence and hidden micro-metastases more correctly than traditional imaging modalities such as CT, MRI, and US. Furthermore, Freeman et al. reported that the overall preoperative accuracy using imaging tests was only around 50%, regardless of the radiological methods used (40). Therefore, we need new methods to predict tumor aggressiveness in HCC replacing traditional criteria based on tumor morphology. Until now, PET/CT could be the strong alternative to be one of the factors for selecting criteria in LDLT for HCC.
Lee et al. showed the possibility of PET/CT as a selecting criteria (5). The advanced HCC patients with PET/CT positivity and tumor total size over 10 cm showed comparable survival in comparison with the patients within the Milan criteria. These criteria could be expanded to all patients with HCC for LDLT. These criteria used both morphologic and biologic factors as a hybrid concept. It could be possible to expand the criteria and predict the prognosis more clearly compared to traditional morphological criteria. We reanalyzed our data using new criteria with PET/CT and total tumor size as National Cancer Center Korea (NCCK) criteria. Among 280 patients (Mar 2005 to May 2013), 164 (58.6%) patients fulfilled the NCCK criteria and 132 patients (47.1%) met the Milan criteria. Five-year overall and DFS rates for patients who met the NCCK criteria showed 85.2% and 84.0%, respectively, and were significantly higher than those beyond the NCCK criteria (0.2% and 44.4%, respectively; P<0.001). The correlation analysis between preoperative imaging tests and pathologic reports demonstrated the better results in the NCCK criteria than those in the Milan criteria (Cohen’s Kappa, 0.850 vs. 0.583, respectively). These new criteria could be the substitution for the traditional criteria in HCC for LDLT. Although PET/CT is not a popular test in HCC for LDLT until now, the usefulness of PET/CT for detecting metastasis and predicting prognosis might be expanded with new tracer and technology.
Conclusions
PET/CT is an important test for the preoperative work-up in patients with HCC for LDLT. According to PET/CT results, we could predict the tumor aggressiveness in especially advanced HCC and detect the hidden malignancies and extrahepatic metastases missed by other imaging modalities. The use of PET/CT for patients with HCC should be expanded in LDLT field, and PET/CT could be the criteria to select the patients with HCC for LDLT replacing the traditional morphologic based criteria.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Chen CL, Fan ST, Lee SG, et al. Living-donor liver transplantation: 12 years of experience in Asia. Transplantation 2003;75:S6-11. [Crossref] [PubMed]
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-9. [Crossref] [PubMed]
- Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001;33:1394-403. [Crossref] [PubMed]
- Lee SG. Asian contribution to living donor liver transplantation. J Gastroenterol Hepatol 2006;21:572-4. [Crossref] [PubMed]
- Lee SD, Kim SH, Kim SK, et al. Clinical Impact of 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in Living Donor Liver Transplantation for Advanced Hepatocellular Carcinoma. Transplantation 2015;99:2142-9. [Crossref] [PubMed]
- Choi HJ, Kim DG, Na GH, et al. Extended criteria for living donor liver transplantation in patients with advanced hepatocellular carcinoma. Transplant Proc 2012;44:399-402. [Crossref] [PubMed]
- Kaido T, Takada Y, Uemoto S. Usefulness of the Kyoto criteria as selection criteria for living donor liver transplantation for hepatocellular carcinoma. Liver Transpl 2010;16:538-40. [PubMed]
- Toso C, Asthana S, Bigam DL, et al. Reassessing selection criteria prior to liver transplantation for hepatocellular carcinoma utilizing the Scientific Registry of Transplant Recipients database. Hepatology 2009;49:832-8. [Crossref] [PubMed]
- Ito T, Takada Y, Ueda M, et al. Expansion of selection criteria for patients with hepatocellular carcinoma in living donor liver transplantation. Liver Transpl 2007;13:1637-44. [Crossref] [PubMed]
- Lee SD, Kim SH, Kim YK, et al. (18)F-FDG-PET/CT predicts early tumor recurrence in living donor liver transplantation for hepatocellular carcinoma. Transpl Int 2013;26:50-60. [Crossref] [PubMed]
- Lee JW, Paeng JC, Kang KW, et al. Prediction of tumor recurrence by 18F-FDG PET in liver transplantation for hepatocellular carcinoma. J Nucl Med 2009;50:682-7. [Crossref] [PubMed]
- Kornberg A, Küpper B, Tannapfel A, et al. Patients with non-[18 F]fludeoxyglucose-avid advanced hepatocellular carcinoma on clinical staging may achieve long-term recurrence-free survival after liver transplantation. Liver Transpl 2012;18:53-61. [Crossref] [PubMed]
- Taketomi A, Sanefuji K, Soejima Y, et al. Impact of des-gamma-carboxy prothrombin and tumor size on the recurrence of hepatocellular carcinoma after living donor liver transplantation. Transplantation 2009;87:531-7. [Crossref] [PubMed]
- Takada Y, Ito T, Ueda M, et al. Living donor liver transplantation for patients with HCC exceeding the Milan criteria: a proposal of expanded criteria. Dig Dis 2007;25:299-302. [Crossref] [PubMed]
- Roayaie S, Schwartz JD, Sung MW, et al. Recurrence of hepatocellular carcinoma after liver transplant: patterns and prognosis. Liver Transpl 2004;10:534-40. [Crossref] [PubMed]
- Kim YI, Paeng JC, Cheon GJ, et al. Prediction of Posttransplantation Recurrence of Hepatocellular Carcinoma Using Metabolic and Volumetric Indices of 18F-FDG PET/CT. J Nucl Med 2016;57:1045-51. [Crossref] [PubMed]
- Kim YK, Lee KW, Cho SY, et al. Usefulness 18F-FDG positron emission tomography/computed tomography for detecting recurrence of hepatocellular carcinoma in posttransplant patients. Liver Transpl 2010;16:767-72. [Crossref] [PubMed]
- Hiraoka A, Hirooka M, Ochi H, et al. Importance of screening for synchronous malignant neoplasms in patients with hepatocellular carcinoma: impact of FDG PET/CT. Liver Int 2013;33:1085-91. [Crossref] [PubMed]
- Mocherla B, Kim J, Roayaie S, et al. FDG PET/CT imaging to rule out extrahepatic metastases before liver transplantation. Clin Nucl Med 2007;32:947-8. [Crossref] [PubMed]
- Peterson MS, Baron RL, Murakami T. Hepatic malignancies: usefulness of acquisition of multiple arterial and portal venous phase images at dynamic gadolinium-enhanced MR imaging. Radiology 1996;201:337-45. [Crossref] [PubMed]
- Shapiro RS, Katz R, Mendelson DS, et al. Detection of hepatocellular carcinoma in cirrhotic patients: sensitivity of CT and ultrasonography. J Ultrasound Med 1996;15:497-502. [PubMed]
- Kim YK, Kim CS, Han YM, et al. Detection of small hepatocellular carcinoma: intraindividual comparison of gadoxetic acid-enhanced MRI at 3.0 and 1.5 T. Invest Radiol 2011;46:383-9. [Crossref] [PubMed]
- Choi BI, Park JH, Kim BH, et al. Small hepatocellular carcinoma: detection with sonography, computed tomography (CT), angiography and Lipiodol-CT. Br J Radiol 1989;62:897-903. [Crossref] [PubMed]
- Miller WJ, Federle MP, Campbell WL. Diagnosis and staging of hepatocellular carcinoma: comparison of CT and sonography in 36 liver transplantation patients. AJR Am J Roentgenol 1991;157:303-6. [Crossref] [PubMed]
- Kim SH, Kim SH, Lee J, et al. Gadoxetic acid-enhanced MRI versus triple-phase MDCT for the preoperative detection of hepatocellular carcinoma. AJR Am J Roentgenol 2009;192:1675-81. [Crossref] [PubMed]
- Joo I, Lee JM. Recent Advances in the Imaging Diagnosis of Hepatocellular Carcinoma: Value of Gadoxetic Acid-Enhanced MRI. Liver Cancer 2016;5:67-87. [Crossref] [PubMed]
- Beyer T, Townsend DW, Brun T, et al. A combined PET/CT scanner for clinical oncology. J Nucl Med 2000;41:1369-79. [PubMed]
- Chen YK, Hsieh DS, Liao CS, et al. Utility of FDG-PET for investigating unexplained serum AFP elevation in patients with suspected hepatocellular carcinoma recurrence. Anticancer Res 2005;25:4719-25. [PubMed]
- Teefey SA, Hildeboldt CC, Dehdashti F, et al. Detection of primary hepatic malignancy in liver transplant candidates: prospective comparison of CT, MR imaging, US, and PET. Radiology 2003;226:533-42. [Crossref] [PubMed]
- Oyama N, Akino H, Kanamaru H, et al. 11C-acetate PET imaging of prostate cancer. J Nucl Med 2002;43:181-6. [PubMed]
- Park JW, Kim JH, Kim SK, et al. A prospective evaluation of 18F-FDG and 11C-acetate PET/CT for detection of primary and metastatic hepatocellular carcinoma. J Nucl Med 2008;49:1912-21. [Crossref] [PubMed]
- Cheung TT, Ho CL, Lo CM, et al. 11C-acetate and 18F-FDG PET/CT for clinical staging and selection of patients with hepatocellular carcinoma for liver transplantation on the basis of Milan criteria: surgeon's perspective. J Nucl Med 2013;54:192-200. [Crossref] [PubMed]
- Pawlik TM, Gleisner AL, Anders RA, et al. Preoperative assessment of hepatocellular carcinoma tumor grade using needle biopsy: implications for transplant eligibility. Ann Surg 2007;245:435-42. [Crossref] [PubMed]
- Otto G, Herber S, Heise M, et al. Response to transarterial chemoembolization as a biological selection criterion for liver transplantation in hepatocellular carcinoma. Liver Transpl 2006;12:1260-7. [Crossref] [PubMed]
- Schwartz M, Dvorchik I, Roayaie S, et al. Liver transplantation for hepatocellular carcinoma: extension of indications based on molecular markers. J Hepatol 2008;49:581-8. [Crossref] [PubMed]
- Shirabe K, Itoh S, Yoshizumi T, et al. The predictors of microvascular invasion in candidates for liver transplantation with hepatocellular carcinoma-with special reference to the serum levels of des-gamma-carboxy prothrombin. J Surg Oncol 2007;95:235-40. [Crossref] [PubMed]
- Kornberg A, Freesmeyer M, Bärthel E, et al. 18F-FDG-uptake of hepatocellular carcinoma on PET predicts microvascular tumor invasion in liver transplant patients. Am J Transplant 2009;9:592-600. [Crossref] [PubMed]
- Yang SH, Suh KS, Lee HW, et al. The role of (18)F-FDG-PET imaging for the selection of liver transplantation candidates among hepatocellular carcinoma patients. Liver Transpl 2006;12:1655-60. [Crossref] [PubMed]
- Bailly M, Venel Y, Orain I, et al. 18F-FDG PET in Liver Transplantation Setting of Hepatocellular Carcinoma: Predicting Histology? Clin Nucl Med 2016;41:e126-9. [Crossref] [PubMed]
- Freeman RB, Mithoefer A, Ruthazer R, et al. Optimizing staging for hepatocellular carcinoma before liver transplantation: A retrospective analysis of the UNOS/OPTN database. Liver Transpl 2006;12:1504-11. [Crossref] [PubMed]


