Nutrition assessment and its effect on various clinical variables among patients undergoing liver transplant
Introduction
Nutrition was included as one of the variables in the original Child and Turcotte [1964] prognostic score (1). Malnutrition is universally present in patients with end stage liver disease (ESLD) undergoing liver transplant (LT) and has been associated with higher morbidity and mortality (2-4).
Nutritional status is one of the variables that was highly correlated with patient’s survival also was not dependent on the disease status, and was therefore potentially reversible (4-7). PEM has been related to various adverse outcomes like decreased graft and patient survival after LT (8). Malnutrition was associated with prolonged ventilator support, longer intensive care unit and hospital stays (6,9).
Nutrition intervention is necessary for a malnourished pre-LT patient’s recovery, and it is cost-effective before LT, as malnourished patients are at high risk for nutritionally mediated complications. It is important to identify and correct nutritional deficiencies in pre-LT patients and provide an optimal nutritional intervention during all phases of LT (10-14).
The accurate nutritional status assessment in ESLD patients is difficult, mainly because of overlap with other complications like fluid retention and hypoproteinemia. Liver disease specifically affects such conventional markers of nutrition like serum proteins levels synthesized by the liver (albumin, transferrin, retinol-binding protein), and immunological dysfunction. Irrespective of these problems in nutrition assessment, diagnosis of malnutrition can be assessed in 20% of patients with compensated liver disease and in >80% with decompensated liver disease (4,8). Despite these findings there is no gold standard for the nutritional status assessment in LT.
Considering the difficulties in nutrition status assessment among ESLD patients and disparity in malnutrition status by different assessment methods, the present study aimed to analyze the nutrition status of patients undergoing LT by using different nutrition assessment tools and associate it with various clinical and prognostic variables of LT.
Methods
Out of five multispecialty hospitals undertaking LT in Delhi and NCR, India, three agreed to participate in the study. All adult (age ≥18 years) patients suffering from ESLD admitted to the participating hospitals for LT were approached; those who gave informed consent were recruited for the study. The nutrition assessment was performed on 54 pre-LT recipients who were available during the study period (September 2013 to June 2015) and underwent living donor LT. Ethical clearance was obtained from institutional ethical committee. Patients were given relevant information through a patient information sheet and written informed consent was taken prior to data collection. Exclusion criteria included the following: (I) patients below 18 years of age as there is a completely different protocol for paediatric LT; (II) patients suffering from acute liver disease as they require emergency transplantation.
Nutrition assessment
Anthropometric measurements
Easily applicable anthropometric measurements include body mass index (BMI), triceps skin-fold (TSF) thickness, and mid upper arm circumference (MUAC) (15). Most of these easily applicable methods are confounded by significant fluid retention in cirrhotics (peripheral edema and ascites). BMI has been criticized for yielding falsely high values, but correction by subtracting estimated amounts of ascites and other fluid collections may compensate for this disadvantage (13). Anthropometric parameters used to assess muscle and fat masses were MUAC, TSF thickness and mid arm muscle circumference (MAMC) by a trained nutrition expert to evaluate nutritional status in patients with chronic liver disease (CLD) (16). BMI for ascites was also used as it is considered more reliable parameter to detect malnutrition in cirrhotic patients than BMI cut-off values. BMI for ascites considers nutrition state with no Ascites, moderate ascites and tense ascites patients, respectively. Peripheral edema and removal of ascites do not affect BMI for ascites diagnostic performance (17).
In the present study anthropometric assessment was performed on 54 pre-LT recipients by MUAC, TSF, MAMC and BMI for ascites.
Biochemical parameters
Various visceral plasma proteins like albumin, prealbumin, retinol-binding protein and 24-hour creatinine excretion are highly affected by the presence of liver disease and inflammatory states, as these are synthesized in liver (18). Biochemical markers of malnutrition include serum albumin concentration and measurements of 24-hour creatinine excretion related to LT patients (19).
Albumin is an important determinant of body’s protein status and an important indicator of liver function; hence we cannot ignore its effect on various clinical parameters. Hence, Albumin levels of 54 LT candidates were also considered for analysis of the nutrition state in the present study.
Bioimpedance analysis (BIA)
BIA assessment was performed on only 20 pre-LT patients because patients refused to undertake this assessment as most of the patients were not fully active and it was not feasible to move the assessment tool multi frequency body composition analyzer (MC-180MA) to reach the patients individually. Information regarding fat mass, fat free mass (FFM), Fat%, muscle mass and phase angle was obtained. BIA measures body composition by two electrical parameters: electrical reactance (Xc) and resistance (R). The phase angle is the impedance vector relative to the R vector, calculated as an arc tangent of the ratio Xc to R transformed to degree (20).
Phase angle is considered as an indicator of membrane integrity and water distribution between intra and extra cellular spaces, body cell mass (BCM) predictor and as a nutrition indicator (21-24). The phase angle of the whole body is similar to the mean phase angle of arms and legs, whereas the trunk has a larger phase angle, it recommended to use phase angle from arms and legs (25). BIA is a precise and non-invasive technique that measures lean body mass and fat stores; but is inaccurate when patients retain fluid. Despite some of the limitations in patients with ascites, BIA is a reliable tool for the determination of BCM in cirrhotic patients with and without ascites (26). Phase angle has been considered to be a prognostic tool in various clinical situations, such as human immuno virus (21), renal disease (27), pulmonary tuberculosis (28), cancer (29), and liver cirrhosis (15,25,30,31).
Considering the emerging importance of phase angle as a nutrition status indicator, the present study used the cut off range from Selberg and Selberg, 2002, i.e., <4.4 (abnormal), 4.4–5.4 (borderline) and >5.4 (normal or healthy). Higher phase angle is seen in healthy individuals (25).
Subjective global assessment (SGA)
SGA approach is the preferred assessment method for LT candidates (6,32-34). SGA was performed on 54 pre-LT recipients. The five features of SGA were analyzed: (I) weight loss before 6 months; (II) dietary intake, duration and degree of abnormal intake (starvation, hypo caloric liquids, full liquid diet, and suboptimal solid diet); (III) gastrointestinal (GI) symptoms like anorexia, nausea, vomiting, and diarrhoea (persisting for more than 2 weeks); (IV) functional capacity history of patients (bedridden to full capacity) and (V) metabolic demands of the patient’s underlying disease state (35). A SGA rank was given which indicates the patient’s nutritional status. These categories are: (I) well nourished; (II) moderate or suspected malnutrition; and (III) severe malnutrition (35). SGA for diagnosing malnutrition in patients with CLD has shown to have high specificity (96%) with a very low sensitivity (22%). However, SGA is proposed as a reliable tool for evaluation of nutritional status in LT patients (33).
Hand grip strength (HG)
HG was the only technique that predicted a significant incidence of major complications in 1 year among undernourished cirrhotic patients. HG is a simple, inexpensive, and effective method to detect malnutrition in cirrhotics because it can identify those patients who are most likely to develop complications (36,37). When compared with SGA, HG lacked specificity. The present study also used HG Strength assessment by Jamar Hand Dynamometer on 54 pre-LT recipients.
Clinical variables
The disease severity of 54 patients undergoing LT was analyzed by CTP grades A, B, C and MELD scores. The various diagnoses of the patients undergoing LT were listed. The levels of ascites were graded as no, mild and tense ascites. After the transplantation, patient stay in the hospital was also considered in three categories: (I) ICU days; (II) ventilator days; (III) hospital days. After one year of transplant, the level of mortality was analyzed by the dead and alive profile of the patients (43 patients). During the transplant the blood product usage was taken into account, that is, PRC (packed RBC) units, FFP (fresh frozen plasma) units, Cryo (cryoprecipitate) units and the blood loss during surgery (33 patients undergoing LT). Patients’ body composition profile was analyzed by body weight, fat mass, fat free mass (FFM), fat%, muscle mass (20 patients undergoing LT).
Statistical analysis
All statistical analysis was performed using the statistical package for social science (SPSS) version 17.0 for Windows (SPSS Inc., Chicago, IL, USA). The kappa test was applied to evaluate the extent of agreement between different nutrition assessment methods. Categorical variables were presented in frequency tables. Associations between categorical variables were evaluated through chi-square tests. Normal variables were presented as mean ± SD and were analyzed by Kruskal Wallis test. Also spearman’s rank correlation was used to know association of HG with ICU days, ventilator days, and hospital stay and blood product usage. An acceptable level of statistical significance was established a priori at P<0.05.
Results
Demographic profile
The demographic profile (Table 1) of patients undergoing LT represents 72.2% were men and 27.8% were females with a mean age of 48.3 years. Majority of the patients were Indians. About 40.7% of the patients were having blood group B+.
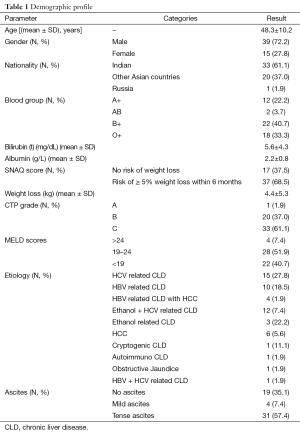
Full table
The data represents 57.4% of patients suffering from tense ascites, low mean albumin levels (2.2 g/L) and high bilirubin levels (5.6 mg/dL). About 98.1% of the patients were not suffering from any food allergy. According to simplified nutrition assessment questionnaire (SNAQ) 68.5% of the patients were having risk of ≥5% weight loss within 6 months (38). The mean weight loss was 4.4 kg before 6 months of transplant.
The CTP scores depicts 61.1% of the patients in CTP grade C and 51.9% of the patients in the MELD range of 19–24. Diagnostically 27.8% and 22.2% of the patients were undergoing LT because of HCV related infections and ethanol related CLD respectively.
Dietary profile
The nutrition profile of the patients before LT in Table 2 showed 88.9% of the patients were on a special diet (modified as per the symptoms), 94.4% of the patients were recommended normal diet (no textural change). Fluid was restricted in 79.7% of the patients and 59.3% of the patients were strictly recommended to restrict the fluid to <1.5 litres. Salt was restricted in about 59.3% of the patients and 25.9% of the patients were having both salt and fluid restriction. All the patients were not having any GI, chewing or dental problem. CAGE questionnaire depicted 31.5% of the patients as alcoholic.
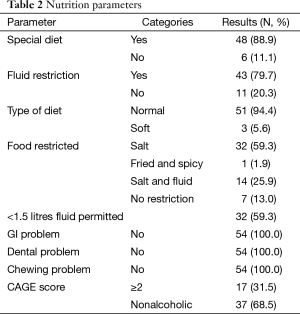
Full table
Nutrition assessment
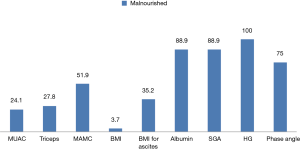
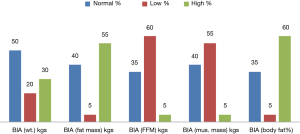
The varied prevalence of nutrition is depicted in Figure 1, which showed HG having the highest prevalence, 100%, SGA and albumin showed 88.9% of malnutrition and phase angle and MAMC showed 75% and 51.9% of patients as malnourished respectively; whereas BMI for ascites showed 35.2%, triceps depicted 27.9% and MUAC showed 24.1% as malnourished. BMI only showed 3.7% of the patients as undernourished. The body composition analysis of 20 patients showed pre-LT recipients had low levels of FFM and muscle mass in more than 50% of the patients and higher body weight, fat mass, and body fat% (Figure 2).
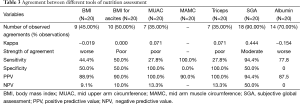
Agreement between different nutrition assessment methods (Table 3) by Kappa was done considering phase angle by BIA body composition analyzer as a gold standard. Phase angle is considered to be a good prognostic tool in various clinical situations. It showed poor or worse agreement with anthropometric tools like BMI, BMI for ascites, MUAC, triceps, MAMC and albumin except with SGA which showed moderate agreement (κ=0.444). SGA showed higher sensitivity and positive predictive value (94.4%) and average specificity and negative predictive value (NPV) of 50%. Table 3 also depicted positive predictive value of 100% by MUAC and triceps whereas 90% by MAMC which depicts chances of predicting more patients as malnourished who actually are normal.
Full table
Nutrition status and various clinical variables
The prevalence of malnutrition by different nutrition assessment tools varied widely according to various clinical factors of pre- and post-LT like indications of LT, CTP grades, MELD scores, degree of ascites, blood units usage during transplantation, blood loss during surgery, ICU, ventilator days and hospital stay, BIA (weight, fat mass, FFM, muscle mass, fat%) and dead and alive status of the patient after LT.
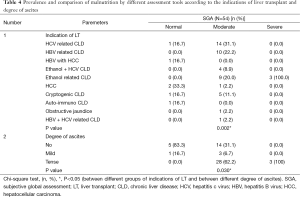
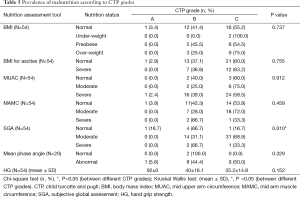
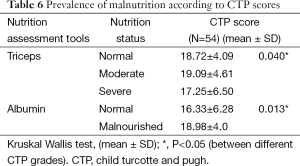
Only SGA out of 9 nutrition assessment tools showed significant association with various indications of LT. Moderate malnutrition was significantly higher (P=0.002) in all the indicators of LT except HCC, HBV + HCC (Table 4). Triceps, albumin and SGA were significantly related to CTP Scores and CTP grades (Tables 5,6). The patients with moderate malnutrition before the transplant were having significantly higher (P=0.010) CTP grades than normal and severe malnutrition by SGA. Triceps and albumin showed significantly higher scores of CTP in malnourished patients undergoing LT (P=0.04, 0.013). There was no relation of MELD prognostic score with nutrition status of patients of undergoing LT.
Full table
Full table
Full table
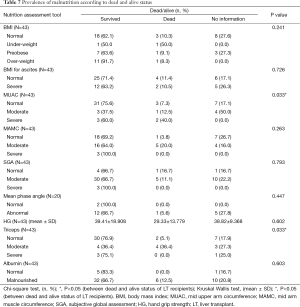
Nutrition status assessment with ascites depicted (Table 4) only SGA was significantly related to degree of ascites, as the patients with moderate and severe malnutrition before the transplant was having significantly higher degree/tense ascites than normal patients (P=0.03). MUAC and Triceps were significantly associated to dead and alive status after LT (Table 7). MUAC and triceps depicted significantly higher survival in normal nutrition status than malnourished patients (P=0.033). Triceps also depicted malnourished patients were having significantly higher deaths than the normal.
Full table
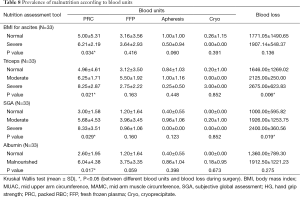
BMI for ascites, triceps, SGA and albumin (P=0.034, 0.021, 0.029 and 0.017) showed use of significantly higher PRC units during the surgery in malnourished patients than the normal patients. Triceps and SGA (P=0.006, 0.019) showed significantly higher blood loss during the surgery in malnourished than the normal patients before LT (Table 8). The present study did not find any significant difference in malnourished patients and ICU, ventilator and hospital days than the normal patients by using various nutrition assessment tools.
Full table
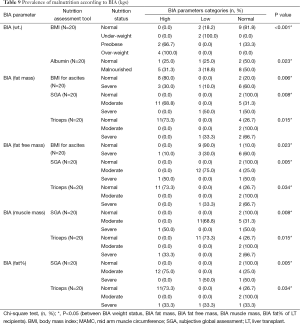
BMI showed overweight patients had significantly higher BIA (weight) with P≤0.001 (Table 9). Also albumin levels significantly showed malnourished having normal weight status (P=0.023). In Table 8 BMI for ascites, SGA and triceps showed normal patients having significantly higher fat mass than malnourished (P=0.006, 0.008 and 0.015). Nutrition assessment by SGA showed significantly lower FFM in malnourished patients than normal (P=0.005). TSF showed significantly higher FFM in patients with normal nutrition status (P=0.034). BMI for ascites showed significantly lower FFM levels in normal patients than severely malnourished (P=0.023). Also lower levels of muscle mass was significantly associated to moderate malnutrition by SGA (P=0.008), whereas significantly lower levels of muscle mass were seen in normal nutrition state by TSF (P=0.015). According to SGA and TSF (P=0.005 and 0.034) assessment, malnourished patients were having significantly higher fat% than the normal (Table 9).
Full table
Discussion
The patients waiting for LT had varied aetiology from viral infections, cancers, alcoholism, autoimmune infections and cryptogenic CLD (39). Ascites, hepatic encephalopathy, portal hypertension, malabsorption and maldigestion of nutrients are common problems of pre-LT patients (40). These problems represent a major challenge in analyzing the nutrition state of the patient awaiting for LT (41).
The present study represents varied prevalence of malnutrition by various nutrition assessment methods ranging from 3.7% to 100%, which showed the disparity and difficulty in analyzing the nutrition state of ESLD patients. BMI and anthropometric measurements showed lower prevalence of malnutrition than phase angle and SGA, whereas muscle strength analysis by HG showed 100% malnutrition (Figure 1).
Earlier studies have shown low agreement among different methods of nutrition assessment tools in ESLD patients considering SGA as a gold standard (42,43). But, the present study depicted low agreement between the different methods of nutrition status assessment except SGA which showed moderate agreement taking phase angle measured by BIA as a gold standard (Table 3). Phase angle has been considered a precise nutrition indicator using body composition parameters (21-24). SGA also showed good sensitivity of about 94.4% and 50% of specificity than the other nutrition assessment tools. MUAC and triceps showed higher positive predictive value, whereas MAMC could not show any κ as there were no observed agreements with the phase angle. HG showed all the patients as malnourished.
The nutrition assessment by various assessment tools also showed prevalence of malnutrition according to various prognostic factors. Malnutrition assessed by different nutrition assessment tools is highly prevalent in ESLD patients irrespective of the varied aetiology of liver disease (12,37,42-45). In the present study, SGA was the only nutrition assessment tool which showed significantly higher malnourished patients in various indications of LT (Table 4). The two prognostic tools for severity of liver disease are CTP and MELD; previous studies had associated higher CTP and MELD grades with malnourished patients (37,46-48). The present study also demonstrated significantly higher prevalence of malnutrition in higher CTP grades by SGA, triceps and albumin (P<0.05) whereas no association was found with MELD scores (Tables 5,6). Malnutrition in patients undergoing LT has been associated to degree/ severity of ascites as a major symptom of liver disease (37,47). In the present study (Table 4) SGA showed malnourished patients were significantly having tense ascites than mild/no (P<0.01). Patients with ESLD undergoing LT are considered to require higher blood product usage like PRC units, cryoprecipitate units, plasma (44,49). The data in Table 8 represents significantly higher blood product usage (PRC units) in malnourished patients by SGA, BMI for ascites, Triceps and albumin (P<0.05). Also the present study depicted significantly higher blood loss during the surgery in malnourished patients as assessed by SGA and triceps (P<0.05). Many studies showed ESLD malnourished patients had lower Survival after LT (6,13,47,50-53). The present study also depicts significantly higher survival in normal patients by MUAC and triceps measurements (Table 7). Unlike other studies (6,13,53) the present study depicted no relation between ICU, ventilator days and hospital stay with the nutrition status of the patients before LT.
Association of malnutrition of ESLD patients with various factors related to prognosis, treatment, and mortality of patients showed pre-LT malnutrition as an important determinant, which is related to various poor surgery outcomes.
Body composition analysis by BIA in LT patients (Table 9) to assess protein malnutrition is considered advantageous (26). CLD patients show lower FFM and muscle mass during body composition analysis (54,55). The present study analyzed the body composition of patients undergoing LT which showed lower FFM, Muscle mass, higher fat mass and body fat% (Figure 2). The patients who were overweight by BIA weight status were significantly having higher BMI and malnourished patients as assessed by albumin were having normal weight status because of water retention (ascites, edema) in ESLD. BIA fat mass was significantly higher in normal patients by triceps and BMI for ascites and SGA (P<0.05) whereas malnourished patients were having significantly higher fat% than normal. Lower levels of FFM and muscle mass were significantly associated to malnourished patients by SGA. Whereas triceps depicts normal patients were having significantly higher FFM than malnourished patients; also triceps showed significantly lower muscle mass in normal patients than malnourished. BMI for ascites showed varied results with significantly lower FFM in normal patients.
Hence, altered body composition is depicted which showed lower FFM, muscle mass and higher weight, fat mass and fat% in Pre-LT patients, which clearly portrays the requirement of nutrition intervention before the LT to attain a correct body composition.
The study used simple, inexpensive tools to analyze the nutrition state of the patients although there are more sophisticated methods like DEXA, K measurements. Simple bedside assessment tools like SGA, anthropometry and BIA can be used to quantify under nutrition according to the recommendation by ESPEN 2006 for liver disease (56,57). But there are certain limitations of BIA method that are, it can only be measured under controlled settings (room temperature, exercise, electrode placement and food intake) (58). Altered hydration status of the patients can affect the assessment readings (59). It is tedious to personalize it for all the patients and ESLD patient may not be in fully active condition to perform this assessment. In the present study also out of 54 patients only 20 were available for the BIA assessment. According to the present analysis only SGA out of 8 methods showed moderate agreement with phase angle of the body. SGA was also significantly associated to majority of the clinical variables like aetiology, CTP grades, degree of ascites, blood product usage (PRC units), blood loss during the surgery, BIA (fat mass, FFM, Muscle mass and body fat%). Also SGA is simple, non-invasive, safe and easily applicable tool for nutrition status assessment. Hence, the present study showed SGA having moderate agreement and was associated with various clinical and prognostic variables of liver transplantation.
Conclusions
Nutrition status assessment is challenging in ESLD patients. Even after considering the problems in nutrition assessment, the importance of nutrition cannot be overlooked in pre-LT patients, as malnutrition is highly associated to various clinical variables of ESLD. SGA showed moderate agreement with the phase angle of the body and was associated with various clinical and prognostic variables of LT.
Acknowledgements
Dr. A.S. Soin: Chief Hepatobiliary and Liver Transplant Surgeon and Chairman of Medanta Institute of Liver Transplantation and Regenerative Medicine, Medanta-The Medicity, Gurgaon, India; Dr. Subash Gupta: Chief Liver Transplant/HPB Surgeon & Director, CLBS, Indraprastha Apollo Hospital, New Delhi, India; and Dr. Vivek Vij: Director, Liver Transplant and GI surgery Fortis Hospital, Noida, for permitting the author to collect information regarding Liver transplant patients from their institute.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by institutional ethics committee of ECR/212/INDT/DL/2014 (No.) and written informed consent was obtained from all patients.
References
- Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg 1964;1:1-85. [PubMed]
- Prijatmoko D, Strauss BJ, Lambert JR, et al. Early detection of protein depletion in alcoholic cirrhosis: role of body composition analysis. Gastroenterology 1993;105:1839-45. [Crossref] [PubMed]
- McCullough AJ, Bugianesi E. Protein-calorie malnutrition and the etiology of cirrhosis. Am J Gastroenterol 1997;92:734-8. [PubMed]
- Lochs H, Plauth M. Liver cirrhosis: rationale and modalities for nutritional support--the European Society of Parenteral and Enteral Nutrition consensus and beyond. Curr Opin Clin Nutr Metab Care 1999;2:345-9. [Crossref] [PubMed]
- Shaw BW Jr, Wood RP, Gordon RD, et al. Influence of selected patient variables and operative blood loss on six-month survival following liver transplantation. Semin Liver Dis 1985;5:385-93. [Crossref] [PubMed]
- Pikul J, Sharpe MD, Lowndes R, et al. Degree of preoperative malnutrition is predictive of postoperative morbidity and mortality in liver transplant recipients. Transplantation 1994;57:469-72. [Crossref] [PubMed]
- Müller MJ. Malnutrition in cirrhosis. J Hepatol 1995;23 Suppl 1:31-5. [PubMed]
- Hasse JM. Nutritional implications of liver transplantation. Henry Ford Hosp Med J 1990;38:235-40. [PubMed]
- Hasse JM, Blue LS, Crippin JS, et al. The effect of nutritional status on length of stay and clinical outcomes following liver transplantation. J Am Diet Assoc 1994;88:143-5.
- Müller MJ, Lautz HU, Plogmann B, et al. Energy expenditure and substrate oxidation in patients with cirrhosis: the impact of cause, clinical staging and nutritional state. Hepatology 1992;15:782-94. [Crossref] [PubMed]
- Nutritional status in cirrhosis. Italian Multicentre Cooperative Project on Nutrition in Liver Cirrhosis. J Hepatol 1994;21:317-25. [PubMed]
- Caregaro L, Alberino F, Amodio P, et al. Malnutrition in alcoholic and virus-related cirrhosis. Am J Clin Nutr 1996;63:602-9. [PubMed]
- Figueiredo FA, Dickson ER, Pasha TM, et al. Utility of standard nutritional parameters in detecting body cell mass depletion in patients with end-stage liver disease. Liver Transpl 2000;6:575-81. [Crossref] [PubMed]
- Sanchez AJ, Aranda-Michel J. Nutrition for the liver transplant patient. Liver Transpl 2006;12:1310-6. [Crossref] [PubMed]
- Selberg O, Böttcher J, Tusch G, et al. Identification of high- and low-risk patients before liver transplantation: a prospective cohort study of nutritional and metabolic parameters in 150 patients. Hepatology 1997;25:652-7. [Crossref] [PubMed]
- Merli M, Nicolini G, Angeloni S, et al. Malnutrition is a risk factor in cirrhotic patients undergoing surgery. Nutrition 2002;18:978-86. [Crossref] [PubMed]
- Campillo B, Richardet JP, Bories PN. Validation of body mass index for the diagnosis of malnutrition in patients with liver cirrhosis. Gastroenterol Clin Biol 2006;30:1137-43. [Crossref] [PubMed]
- Crawford DH, Cuneo RC, Shepherd RW. Pathogenesis and assessment of malnutrition in liver disease. J Gastroenterol Hepatol 1993;8:89-94. [Crossref] [PubMed]
- Pirlich M, Selberg O, Böker K, et al. The creatinine approach to estimate skeletal muscle mass in patients with cirrhosis. Hepatology 1996;24:1422-7. [Crossref] [PubMed]
- Baumgartner RN, Chumlea WC, Roche AF. Bioelectric impedance phase angle and body composition. Am J Clin Nutr 1988;48:16-23. [PubMed]
- Schwenk A, Beisenherz A, Römer K, et al. Phase angle from bioelectrical impedance analysis remains an independent predictive marker in HIV-infected patients in the era of highly active antiretroviral treatment. Am J Clin Nutr 2000;72:496-501. [PubMed]
- Maggiore Q, Nigrelli S, Ciccarelli C, et al. Nutritional and prognostic correlates of bioimpedance indexes in hemodialysis patients. Kidney Int 1996;50:2103-8. [Crossref] [PubMed]
- Pupim LB, Kent P, Ikizler TA. Bioelectrical impedance analysis in dialysis patients. Miner Electrolyte Metab 1999;25:400-6. [Crossref] [PubMed]
- Nagano M, Suita S, Yamanouchi T. The validity of bioelectrical impedance phase angle for nutritional assessment in children. J Pediatr Surg 2000;35:1035-9. [Crossref] [PubMed]
- Selberg O, Selberg D. Norms and correlates of bioimpedance phase angle in healthy human subjects, hospitalized patients, and patients with liver cirrhosis. Eur J Appl Physiol 2002;86:509-16. [Crossref] [PubMed]
- Pirlich M, Schütz T, Spachos T, et al. Bioelectrical impedance analysis is a useful bedside technique to assess malnutrition in cirrhotic patients with and without ascites. Hepatology 2000;32:1208-15. [Crossref] [PubMed]
- Johansen KL, Kaysen GA, Young BS, et al. Longitudinal study of nutritional status, body composition, and physical function in hemodialysis patients. Am J Clin Nutr 2003;77:842-6. [PubMed]
- Van Lettow M, Kumwenda JJ, Harries AD, et al. Malnutrition and the severity of lung disease in adults with pulmonary tuberculosis in Malawi. Int J Tuberc Lung Dis 2004;8:211-7. [PubMed]
- Toso S, Piccoli A, Gusella M, et al. Bioimpedance vector pattern in cancer patients without disease versus locally advanced or disseminated disease. Nutrition 2003;19:510-4. [Crossref] [PubMed]
- Wagner D, Adunka C, Kniepeiss D, et al. Serum albumin, subjective global assessment, body mass index and the bioimpedance analysis in the assessment of malnutrition in patients up to 15 years after liver transplantation. Clin Transplant 2011;25:E396-400. [Crossref] [PubMed]
- Fernandes SA, Bassani L, Nunes FF, et al. Nutritional assessment in patients with cirrhosis. Arq Gastroenterol 2012;49:19-27. [PubMed]
- Hasse J. Role of the dietitian in the nutrition management of adults after liver transplantation. J Am Diet Assoc 1991;91:473-6. [PubMed]
- Hasse JM. Nutrition considerations in liver transplantation. Top ClinNutr 1992;7:24-33. [Crossref]
- Nompleggi DJ, Bonkovsky HL. Nutritional supplementation in chronic liver disease: an analytical review. Hepatology 1994;19:518-33. [Crossref] [PubMed]
- Detsky AS, McLaughlin JR, Baker JP, et al. What is subjective global assessment of nutritional status? JPEN J Parenter Enteral Nutr 1987;11:8-13. [Crossref] [PubMed]
- Alvares-da-Silva MR, Reverbel da Silveira T. Comparison between handgrip strength, subjective global assessment, and prognostic nutritional index in assessing malnutrition and predicting clinical outcome in cirrhotic outpatients. Nutrition 2005;21:113-7. [Crossref] [PubMed]
- Ferreira LG, Anastácio LR, Lima AS, et al. Assessment of nutritional status of patients waiting for liver transplantation. Clin Transplant 2011;25:248-54. [Crossref] [PubMed]
- Wilson MM, Thomas DR, Rubenstein LZ, et al. Appetite assessment: simple appetite questionnaire predicts weight loss in community-dwelling adults and nursing home residents. Am J Clin Nutr 2005;82:1074-81. [PubMed]
- Harrison TR. Principles of internal medicine. 16th ed. 2005:1301-2608.
- CCFA fact sheet news from the IBD help centre. liver disease and IBD. Available online: http://www.ccfa.org/resources/liver-disease-and-ibd.html?referrer
- Marsano L, McClain CJ. Nutrition and alcoholic liver disease. JPEN J Parenter Enteral Nutr 1991;15:337-44. [Crossref] [PubMed]
- Roongpisuthipong C, Sobhonslidsuk A, Nantiruj K, et al. Nutritional assessment in various stages of liver cirrhosis. Nutrition 2001;17:761-5. [Crossref] [PubMed]
- Stephenson GR, Moretti EW, El-Moalem H, et al. Malnutrition in liver transplant patients: preoperative subjective global assessment is predictive of outcome after liver transplantation. Transplantation 2001;72:666-70. [Crossref] [PubMed]
- Kyle UG, Kossovsky MP, Karsegard VL, et al. Comparison of tools for nutritional assessment and screening at hospital admission: a population study. Clin Nutr 2006;25:409-17. [Crossref] [PubMed]
- Ferreira LG, Anastácio LR, Lima AS, et al. Malnutrition and inadequate food intake of patients in the waiting list for liver transplant. Rev Assoc Med Bras 2009;55:389-93. [Crossref] [PubMed]
- Shahid M, Johnson J, Nightingale P, et al. Nutritional markers in liver allograft recipients. Transplantation 2005;79:359-62. [Crossref] [PubMed]
- Gunsar F, Raimondo ML, Jones S, et al. Nutritional status and prognosis in cirrhotic patients. Aliment Pharmacol Ther 2006;24:563-72. [Crossref] [PubMed]
- Figueiredo FA, Perez RM, Freitas MM, et al. Comparison of three methods of nutritional assessment in liver cirrhosis: subjective global assessment, traditional nutritional parameters, and body composition analysis. J Gastroenterol 2006;41:476-82. [Crossref] [PubMed]
- Mohanka R., Yadav S.K, Bakshi N, et al. Nutrition status using subjective global assessment (SGA) independently predicts outcome of patients waiting for living donor liver transplant Liver Transplantation 2014;20:S353-4. (abstract).
- Harrison J, McKiernan J, Neuberger JM. A prospective study on the effect of recipient nutritional status on outcome in liver transplantation. Transpl Int 1997;10:369-74. [Crossref] [PubMed]
- Shields PL, Tang H, Neuberger JM, et al. Poor outcome in patients with diabetes mellitus undergoing liver transplantation. Transplantation 1999;68:530-5. [Crossref] [PubMed]
- Alberino F, Gatta A, Amodio P, et al. Nutrition and survival in patients with liver cirrhosis. Nutrition 2001;17:445-50. [Crossref] [PubMed]
- Merli M, Giusto M, Gentili F, et al. Nutritional status: its influence on the outcome of patients undergoing liver transplantation. Liver Int 2010;30:208-14. [Crossref] [PubMed]
- Kyle UG., Genton L, Mentha G, et al. Reliable bioelectrical impedance analysis estimate of fat-free mass in liver, lung, and heart transplant patients. JPEN J Parenter Enteral Nutr 2001;25:45-51. [Crossref] [PubMed]
- Kaido T, Mori A, Oike F, et al. Impact of pretransplant nutritional status in patients undergoing liver transplantation. Hepatogastroenterology 2010;57:1489-92. [PubMed]
- Plauth M, Cabré E, Riggio O, et al. ESPEN Guidelines on Enteral Nutrition: Liver disease. Clin Nutr 2006;25:285-94. [Crossref] [PubMed]
- Weimann A, Braga M, Harsanyi L, et al. ESPEN Guidelines on Enteral Nutrition: Surgery including organ transplantation. Clin Nutr 2006;25:224-44. [Crossref] [PubMed]
- Kushner RF, Gudivaka R, Schoeller DA. Clinical characteristics influencing bioelectrical impedance analysis measurements. Am J Clin Nutr 1996;64:423S-427S. [PubMed]
- Steiner MC, Barton RL, Singh SJ, et al. Bedside methods versus dual energy X-ray absorptiometry for body composition measurement in COPD. Eur Respir J 2002;19:626-31. [Crossref] [PubMed]


